Middle East and Africa e-Clinical Solutions Market Analysis and Size
The Middle East and Africa e-clinical solutions market is fragmented, as it consists of many global players such as Oracle, IQVIA Inc., Dassault Systemes, and Clario among others. The presence of these companies produces competitive prices for systems services and software across the region. Due to the presence of these players at regional and international levels, suppliers and manufacturers offer products with different specifications and characteristics in all budgets. The growing adoption of electronic data capture (EDC) systems and cloud-based is driving the market growth. Additionally, the growing focus on patient-centric clinical trials is expected to drive market growth.
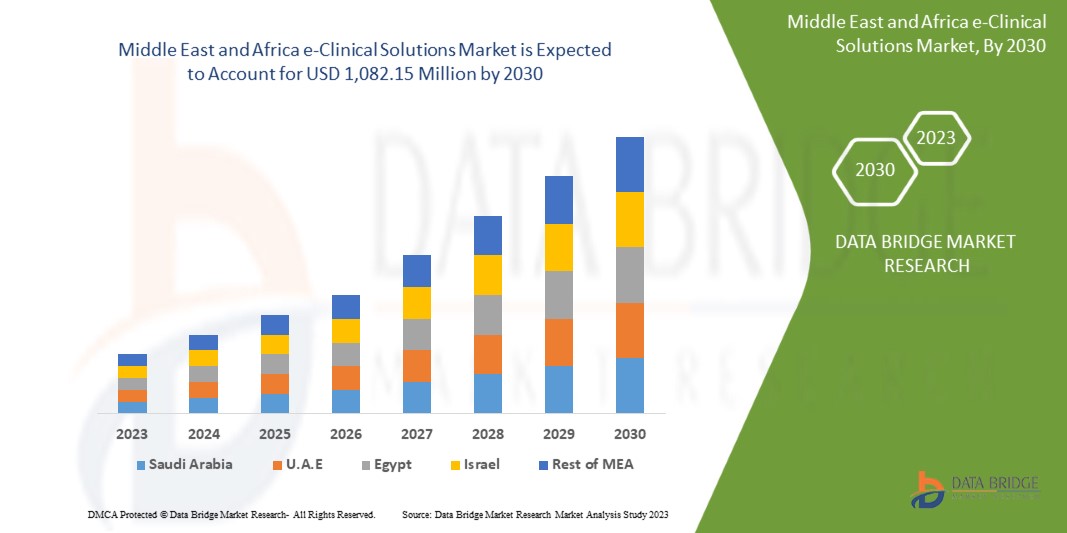
Data Bridge Market Research analyzes that the Middle East and Africa e-clinical solutions market is expected to reach a value of USD 1,082.15 million by 2030, at a CAGR of 11.9% during the forecast period. This market report also covers pricing analysis and technological advancements in depth.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 – 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File Systems, Regulatory Information Management Solutions, and Others), Delivery Mode (Web-Hosted (On- Demand) Solutions, Licensed Enterprise (On-Premises) Solutions, and Cloud-Based (SAAS) Solutions), Clinical Trial Phase (Phase I, Phase II, Phase III, and Phase IV), Organization Size (Small & Medium and Large), User Device (Desktop, Tablet, Handheld PDA Device, Smart Phone, and Others), End User (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, Consulting Service Companies, Medical Device Manufacturers, Hospitals, and Academic Research Institutes) |
|
Countries Covered |
South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and the Rest of the Middle East and Africa |
|
Market Players Covered |
Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, RealTime Software Solutions LLC, Quretec, Research Manager, Datatrack Int., and IQVIA Inc.
|
Market Definition
The goal of e-clinical solutions is to revolutionize the field of clinical research. The clinical management organization is trying to improve the effectiveness, efficiency, and accessibility of clinical research data to treat patients more quickly. The idea behind the creation of eClinical Solutions was to pinpoint the problems with clinical research data, fix them, and offer creative solutions to make clinical trial data useful and simple to obtain, as well as to facilitate the standardization, reporting, and operation of the clinical research domain. The rising adoption and growing focus on e-clinical solutions and software are expected to drive market growth.
The e-clinical solutions, such as electronic data capture (EDC), electronic patient-reported outcomes (ePRO), clinical trial management systems (CTMS), and electronic trial master file (eTMF) systems, are software programs used in clinical research to manage clinical data. However, strict regulations and standards for the approval of clinical trial studies and product approvals are expected to restrain market growth.
Middle East and Africa e-Clinical Solutions Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of this is discussed in detail below:
DRIVERS
Increasing R&D activities and corresponding expenditure
Research & development is crucial in all industries, including life science and healthcare. The life science industry carries out extensive research and development to generate revenue and often brings results that embrace saving or enhancing a patient's life. Drug development requires going through clinical trials to check clinical data analysis and review its study and new data insights.
The e-clinical solutions are used for comprehensive data review, automating the data transformation process, and analytics to accelerate timelines. Pharmaceutical and biopharmaceutical companies conduct many clinical trials to complete successful drug or biologic development. Pharmaceutical and biopharmaceutical companies have increased their focus on research and development, raising the demand for e-clinical solutions.
Improved healthcare infrastructure and establishment of advanced laboratories
Infrastructure is a key pillar supporting the fundamental aim of promoting improved standards of care and well-being for all patients, together with a good healthcare system experience. In parallel, the healthcare system and staff must support effective health promotion, prevention, and self-care of the whole population.
Infrastructure must integrate the hospital, as the acute and in-patient care center, into the broader healthcare system. It should facilitate the seven domains of quality patient experience, effectiveness, efficiency, timeliness, safety, equity, and sustainability. Infrastructure includes the built environment and supporting elements such as equipment, access, information technology (IT), systems and processes, sustainability initiatives, and staff. This improved healthcare infrastructure and staff more opportunities for e-clinical solutions to grow and give better results.
OPPORTUNITY
Increasing investments by various governments in clinical trials
Clinical trial funding comes from various sources, including the government, commercial investors, nonprofit organizations, academic institutions, and other research organizations. Historically, the National Institutes of Health (NIH) has made the biggest government investments in fundamental drug development research. The defense advanced research agency (DARPA) has also contributed to the discovery stage by accepting a few relatively high-risk biologic initiatives. State governments are also increasingly taking the lead in this area, partly due to the public's frustration with the lengthy discovery process. Thus, government initiatives and investments toward discovering new drugs with trial studies are expected to create an opportunity for market growth.
RESTRAINT / CHALLENGE
Data Safety and Privacy issues
Information systems are heavily concerned with privacy and security. Access to individual health information is made possible by digitalizing healthcare services from any electronic device with an internet connection anywhere globally. Users typically are unaware of how their data is handled. Healthcare data on the cloud has caught the attention of hackers, who target systems to launch attacks and steal sensitive data in exchange for financial benefit.
Data breaches have been common since 2019, over 50 million electronic medical records have been hacked in 2021, and the number of security breaches was predicted to increase every year. The creation of inter-jurisdictional data-sharing agreements and the storage and manipulation of data holdings are significantly hampered by concerns about personal privacy and information confidentiality and the recent enactment of privacy and confidentiality legislation across the provinces and territories (exceptionally patient records). Thus, it is a restraint for the Middle East and Africa e-clinical solutions market and is expected to hamper market growth.
Post-COVID-19 Impact on the Middle East and Africa e-Clinical Solutions Market
COVID-19 positively impacted the Middle East and Africa e-clinical solutions market. The lockdown restriction led to the emergence of various opportunities such as drug development, EMR and EHR application installation, and many others.
However, increasing government support and advanced and innovative techniques are expected to provide lucrative opportunities for market growth. Moreover, increasing partnerships, acquisitions, and collaboration among market players are expected to further fuel market growth. Also, the growth has been high since the market opened after COVID-19, and it is expected that there will be considerable growth in the sector. The market players are conducting multiple activities to improve the trial study techniques. With this, the companies will bring advancement and innovation to the market.
Recent Developments
- In April 2023, the Medidata subsidiary of Dassault Systèmes announced Lambda Therapeutics is deploying Medidata's cloud-based clinical products, Rave EDC, Rave RTSM, and Rave Imaging, according to a statement from Dassault Systèmes subsidiary Medidata. Automating and optimizing data management operations and securely delivering higher-quality data for quicker insights will further improve clinical trial productivity. This has aided the business in promoting its offerings across the globe
- In March 2023, Clario has launched a cloud-based Image Viewer tool that helps the Sponsors and CROs to see the images of their clinical trials. Previously, several organizations had to participate in the image transfer procedure to see photos for a clinical trial. This complicated the already risky process and increased the possibility of delays and mistakes. This has aided the business in growing its service offering
Middle East and Africa e-Clinical Solutions Market Scope
The Middle East and Africa e-clinical solutions market is segmented into six notable segments based on product, delivery mode, clinical trial phase, organization size, user device, and end user. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and insights to help them make strategic decisions for identifying core market applications.
Product
- Electronic Data Capture and Clinical Data Management Systems
- Clinical Trial Management Systems
- Clinical Analytics Platforms
- Care Coordination Medical Record (CCMR)
- Randomization and Trial Supply Management
- Clinical Data Integration Platforms
- Electronic Clinical Outcome Assessment Solutions
- Safety Solutions
- Electronic Trial Master File Systems
- Regulatory Information Management Solutions
- Others
Based on product, the market is segmented into electronic data capture and clinical data management, clinical trial management systems, clinical analytics platforms, care coordination medical record (CCMR), randomization and trial supply management, clinical data integration platforms, electronic clinical outcome assessment solutions, safety solutions, electronic trial master file systems, regulatory information management solutions and others.
Delivery Mode
- Web-Hosted (On-Demand) Solutions
- Licensed Enterprise (On-Premises) Solutions
- Cloud-based (SAAS) Solutions
Based on delivery mode, the market is segmented into web-hosted (on-demand) solutions, licensed enterprise (on-premises) solutions, and cloud-based (SAAS) solutions.
Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Based on clinical trial phase, the market is segmented into Phase I, Phase II, Phase III, and Phase IV.
Organization Size
- Small & Medium
- Large
Based on organization size, the market is segmented into small & medium, and large.
User Device
- Desktop
- Tablet
- Handheld PDA Device
- Smart Phone
- Others
Based on user device, the market is segmented into desktop, tablet, handheld PDA device, smart phone, and others.
End User
- Pharmaceutical and Biopharmaceutical companies
- Contract Research Organizations
- Consulting Service Companies
- Medical Device Manufacturers
- Hospitals
- Academic Research Institutes
Based on end user, the market is segmented into pharmaceutical and biopharmaceutical companies, contract research organizations, consulting service companies, medical device manufacturers, hospitals, and academic research institutes.
Middle East and Africa e-Clinical Solutions Market Regional Analysis/Insights
The Middle East and Africa e-clinical solutions market is analyzed, and market size information is p based on product, delivery mode, clinical trial phase, organization size, user device, and end user.
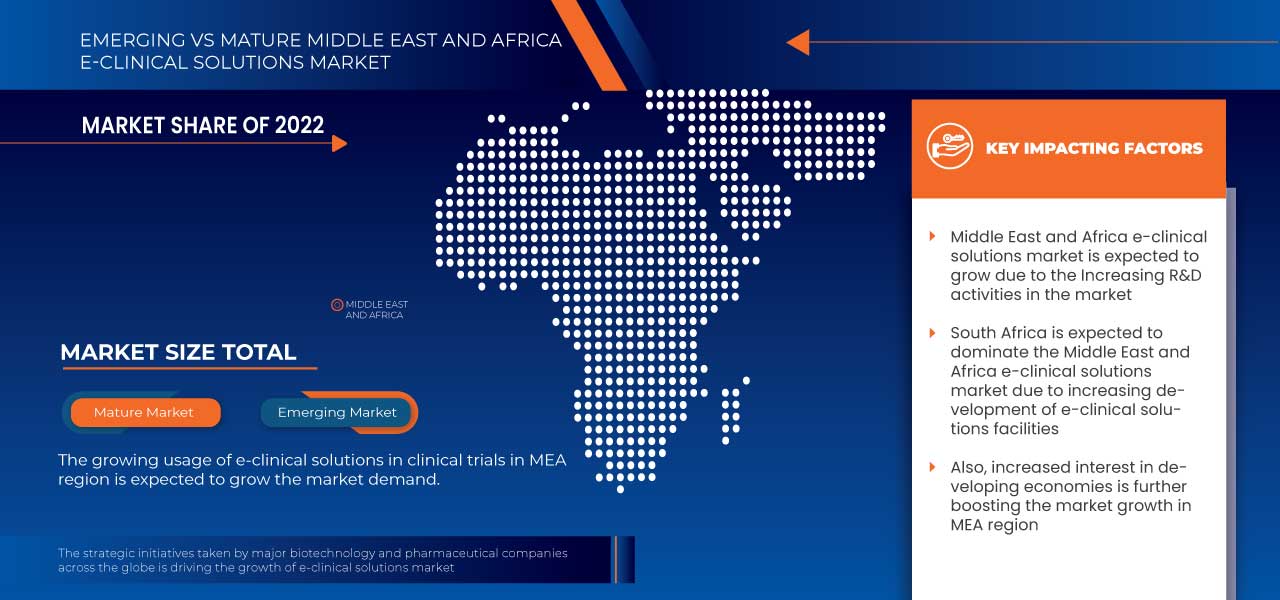
The countries covered in this market report are South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and the Rest of the Middle East and Africa.
South Africa is expected to dominate the Middle East and Africa region due to the rising adoption of electronic data capture systems and cloud-based solutions.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Asia-Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East and Africa e-Clinical Solutions Market Share Analysis
The Middle East and Africa e-clinical solutions market competitive landscape provides details of the competitor. Details include company overview, financials, revenue generated, market potential, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus related to the market.
Some of the major market players operating in the Middle East and Africa e- clinical solutions market are Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, and RealTime Software Solutions among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S FIVE FORCES MODEL
4.2 ECOSYSTEMS ANALYSIS OF E CLINICAL SOLUTIONS
4.3 USE CASES
5 VALUE CHAIN ANALYSIS
6 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 THE GROWING USAGE OF E-CLINICAL SOLUTIONS IN CLINICAL TRIALS
7.1.2 INCREASING R&D ACTIVITIES AND CORRESPONDING EXPENDITURE
7.1.3 STRATEGIC INITIATIVES TAKEN BY MAJOR BIOTECHNOLOGY AND PHARMACEUTICAL COMPANIES
7.1.4 IMPROVED HEALTHCARE INFRASTRUCTURE AND ESTABLISHMENT OF ADVANCED LABORATORIES
7.2 RESTRAINTS
7.2.1 DATA SAFETY AND PRIVACY ISSUES
7.2.2 LACK OF SKILLED PROFESSIONALS
7.2.3 LIMITED ADOPTION OF E-CLINICAL SOLUTIONS IN EMERGING COUNTRIES DUE TO BUDGETARY CONSTRAINTS AND POOR MANAGEMENT POLICIES
7.3 OPPORTUNITIES
7.3.1 RISING ADOPTION OF ELECTRONIC DATA CAPTURE (EDC) SYSTEMS AND CLOUD-BASED SOLUTIONS
7.3.2 INCREASING INVESTMENTS BY VARIOUS GOVERNMENTS IN CLINICAL TRIALS
7.3.3 GROWING FOCUS ON PATIENT-CENTRIC CLINICAL TRIALS
7.4 CHALLENGES
7.4.1 REGULATORY CHALLENGES ASSOCIATED WITH E-CLINICAL SOLUTIONS
7.4.2 HIGH IMPLEMENTATION COSTS
8 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS
8.3 RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT
8.4 CLINICAL TRIAL MANAGEMENT SYSTEMS
8.5 ELECTRONIC TRIAL MASTER FILE SYSTEMS
8.6 ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS
8.7 SAFETY SOLUTIONS
8.8 REGULATORY INFORMATION MANAGEMENT SOLUTIONS
8.9 CLINICAL DATA INTEGRATION PLATFORMS
8.1 CLINICAL ANALYTICS PLATFORMS
8.11 CARE COORDINATION MEDICAL RECORD (CCMR)
8.12 OTHERS
9 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE
9.1 OVERVIEW
9.2 WEB-HOSTED (ON-DEMAND) SOLUTIONS
9.3 CLOUD-BASED (SAAS) SOLUTIONS
9.4 LICENSED ENTERPRISE (ON-PREMISES) SOLUTIONS
10 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE
10.1 OVERVIEW
10.2 PHASE III
10.3 PHASE I
10.4 PHASE II
10.5 PHASE IV
11 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE
11.1 OVERVIEW
11.2 MEDIUM AND SMALL
11.3 LARGE
12 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE
12.1 OVERVIEW
12.2 DESKTOP
12.3 TABLET
12.4 SMART PHONE
12.5 HANDHELD PDA DEVICE
12.6 OTHERS
13 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER
13.1 OVERVIEW
13.2 CONTRACT RESEARCH ORGANIZATIONS
13.3 PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES
13.4 MEDICAL DEVICE MANUFACTURERS
13.5 HOSPITAL
13.6 CONSULTING SERVICE COMPANIES
13.7 ACADEMIC RESEARCH INSTITUTES
14 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY REGION
14.1 MIDDLE EAST AND AFRICA
14.1.1 SOUTH AFRICA
14.1.2 SAUDI ARABIA
14.1.3 U.A.E.
14.1.4 EGYPT
14.1.5 ISRAEL
14.1.6 REST OF MIDDLE EAST AND AFRICA
15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 ORACLE
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 DASSAULT SYSTEMES
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 CLARIO
17.3.1 COMPANY SNAPSHOT
17.3.2 COMPANY SHARE ANALYSIS
17.3.3 PRODUCT PORTFOLIO
17.3.4 RECENT DEVELOPMENT
17.4 VEEVA SYSTEMS
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.5 ARISMIDDLE EAST & AFRICA
17.5.1 COMPANY SNAPSHOT
17.5.2 COMPANY SHARE ANALYSIS
17.5.3 PRODUCT PORTFOLIO
17.5.4 RECENT DEVELOPMENTS
17.6 4G CLINICAL
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 ADVARRA.
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENT
17.8 ANJU
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENTS
17.9 CASTOR.
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENTS
17.1 DATATRAK INT.
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.11 ECLINICAL SOLUTIONS LLC.
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENT
17.12 IQVIA INC
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 MAXISIT
17.13.1 COMPANY SNAPSHOT
17.13.2 PRODUCT PORTFOLIO
17.13.3 RECENT DEVELOPMENT
17.14 MEDNET
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 MEDRIO, INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENTS
17.16 MERATIVE
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENT
17.17 OPENCLINICA, LLC
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENT
17.18 PAREXEL INTERNATIONAL CORPORATION
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENT
17.19 QURETEC
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 REALTIME SOFTWARE SOLUTIONS, LLC
17.20.1 COMPANY SNAPSHOT
17.20.2 PRODUCT PORTFOLIO
17.20.3 RECENT DEVELOPMENT
17.21 RESEARCH MANAGER
17.21.1 COMPANY SNAPSHOT
17.21.2 PRODUCT PORTFOLIO
17.21.3 RECENT DEVELOPMENT
17.22 SAAMA TECHNOLOGIES, LLC
17.22.1 COMPANY SNAPSHOT
17.22.2 PRODUCT PORTFOLIO
17.22.3 RECENT DEVELOPMENT
17.23 SIGNANT HEALTH
17.23.1 COMPANY SNAPSHOT
17.23.2 PRODUCT PORTFOLIO
17.23.3 RECENT DEVELOPMENT
17.24 Y-PRIME, LLC.
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
List of Table
TABLE 1 THE COSTS OF IMPLEMENTATION ARE AS FOLLOWS, ACCORDING TO SIMPLETRIALS:
TABLE 2 ACCORDING TO ECLINICAL WORKS – ECLINICAL OFFERS TWO ENTERPRISE PRICING PACKAGES:
TABLE 3 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA CLINICAL TRIAL MANAGEMENT SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA ELECTRONIC TRIAL MASTER FILE SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA SAFETY SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA REGULATORY INFORMATION MANAGEMENT SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CLINICAL DATA INTEGRATION PLATFORMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CLINICAL ANALYTICS PLATFORMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA CARE COORDINATION MEDICAL RECORD (CCMR) IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA OTHERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WEB-HOSTED (ON-DEMAND) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA CLOUD-BASED (SAAS) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA LICENSED ENTERPRISE (ON-PREMISES) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA PHASE III IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA PHASE I IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA PHASE II IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA PHASE IV IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA MEDIUM AND SMALL IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA LARGE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA DESKTOP IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA TABLET IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA SMART PHONE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HANDHELD PDA DEVICE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA OTHERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA CONTRACT RESEARCH ORGANIZATIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA MEDICAL DEVICE MANUFACTURERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITAL IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA CONSULTING SERVICE COMPANIES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA ACADEMIC RESEARCH INSTITUTES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 41 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 42 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 43 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 44 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 45 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 46 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 49 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 50 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 51 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 52 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 53 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 55 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 56 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 57 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 58 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 59 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 60 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 61 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 62 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 63 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 64 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 EGYPT E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 66 EGYPT E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 67 EGYPT E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 68 EGYPT E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 69 EGYPT E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 70 EGYPT E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 72 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 73 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 74 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 75 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 76 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 REST OF MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA E- CLINICAL SOLUTIONS MARKET: SEGMENTATION
FIGURE 11 THE GROWING USAGE OF E-CLINICAL SOLUTIONS IN CLINICAL TRIALS AS WELL AS INCREASING R&D ACTIVITIES AND CORRESPONDING EXPENDITURE, IS EXPECTED TO DRIVE THE GROWTH OF THE MIDDLE EAST & AFRICA E- CLINICAL SOLUTIONS MARKET FROM 2023 TO 2030
FIGURE 12 PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET
FIGURE 14 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, 2022
FIGURE 15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 16 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 17 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 18 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, 2022
FIGURE 19 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, 2023-2030 (USD MILLION)
FIGURE 20 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, CAGR (2023-2030)
FIGURE 21 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, LIFELINE CURVE
FIGURE 22 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, 2022
FIGURE 23 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, 2023-2030 (USD MILLION)
FIGURE 24 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, CAGR (2023-2030)
FIGURE 25 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, LIFELINE CURVE
FIGURE 26 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, 2022
FIGURE 27 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, 2023-2030 (USD MILLION)
FIGURE 28 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, CAGR (2023-2030)
FIGURE 29 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, LIFELINE CURVE
FIGURE 30 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, 2022
FIGURE 31 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, 2023-2030 (USD MILLION)
FIGURE 32 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, CAGR (2023-2030)
FIGURE 33 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, LIFELINE CURVE
FIGURE 34 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, 2022
FIGURE 35 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 36 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 37 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: SNAPSHOT (2022)
FIGURE 39 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2022)
FIGURE 40 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: PRODUCT (2023-2030)
FIGURE 43 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.