Middle East And Africa Pemphigus Vulgaris Market
Market Size in USD Million
CAGR :
% 
 USD
20.88 Million
USD
36.30 Million
2024
2032
USD
20.88 Million
USD
36.30 Million
2024
2032
| 2025 –2032 | |
| USD 20.88 Million | |
| USD 36.30 Million | |
|
|
|
|
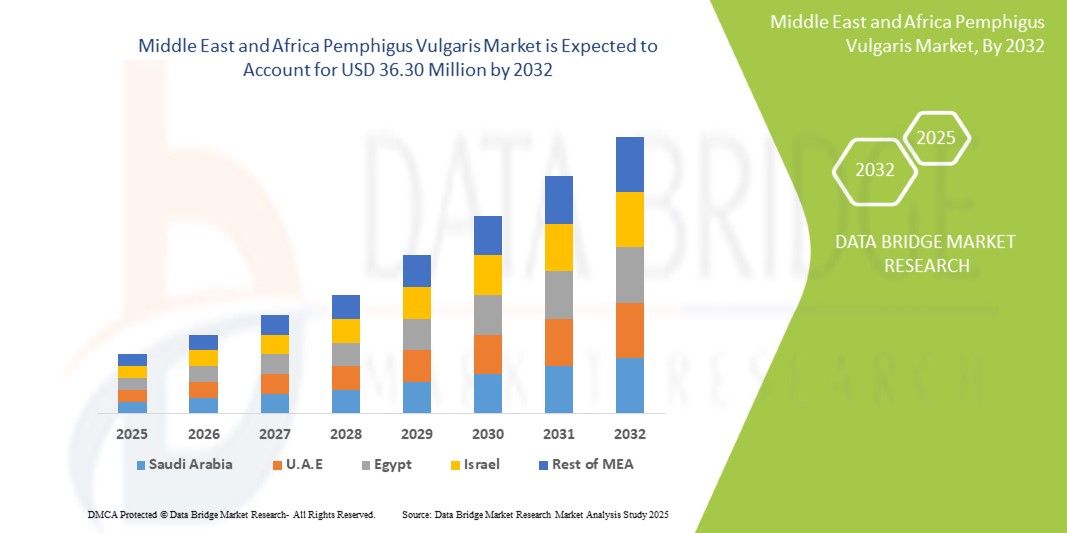
Pemphigus Vulgaris Market Size
- The Middle East and Africa pemphigus vulgaris market size was valued at USD 20.88 million in 2024 and is expected to reach USD 36.30 million by 2032, at a CAGR of 7.3% during the forecast period
- The market growth is largely fueled by rising prevalence of pemphigus vulgaris, increasing awareness about autoimmune skin diseases, advancements in diagnostic techniques, and growing investments in research and development for effective treatment options
- Furthermore, the market is expected to witness continued expansion due to improved healthcare infrastructure, increasing patient access to advanced therapies, supportive government initiatives, and a growing focus on biologics and targeted treatments for managing pemphigus vulgaris more effectively
Pemphigus Vulgaris Market Analysis
- The market is driven by the rising incidence of pemphigus vulgaris, growing awareness of autoimmune diseases, and advancements in biologic therapies, leading to increased demand for effective treatment options and improved patient outcomes regionally
- Key factors influencing market growth include expanding healthcare infrastructure, rising healthcare expenditure, government support for rare disease research, and ongoing clinical trials focused on novel therapeutics targeting pemphigus vulgaris management
- Saudi Arabia is expected to dominates the pemphigus vulgaris market with a 32.74% revenue share in 2025, supported by advanced healthcare infrastructure, high patient awareness, robust research and development activities, widespread availability of biologic therapies, and strong government initiatives promoting rare disease treatment
- Saudi Arabia is expected to be the fastest-growing region in the pemphigus vulgaris market during the forecast period, fueled by increasing healthcare investments, growing patient population, rising awareness, improved diagnostic facilities, and expanding access to advanced treatments across emerging economies.
- The treatment segment is expected to dominate the pemphigus vulgaris market with a 74.16% share in 2025, driven by increasing demand for biologic therapies, rising prevalence of autoimmune diseases, advancements in treatment options, and growing patient preference for effective, targeted therapies.
Report Scope and Pemphigus Vulgaris Market Segmentation
|
Attributes |
Pemphigus Vulgaris Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Middle East and Africa
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Pemphigus Vulgaris Market Trends
“Rising Prevalence of Autoimmune Disorders”
- The increasing incidence of autoimmune disorders is expanding the patient base, driving higher demand for diagnostics, therapies, and healthcare services, thereby significantly fueling market growth regionally
- Market players are investing heavily in research and development to create innovative biologic and targeted treatments, responding to the growing autoimmune disease burden
- Rising awareness and improved diagnostic capabilities are boosting early detection, leading to increased treatment adoption and expanding the overall market size
- The expanding autoimmune disorder market attracts substantial investments from pharmaceutical companies and governments, supporting infrastructure and new product launches that enhance market competitiveness
- Increasing prevalence in emerging economies opens new growth opportunities, prompting market expansion through improved healthcare access and rising healthcare expenditure in these regions. sustainability
Pemphigus Vulgaris Market Dynamics
Driver
“Emerging Treatment Options for Pemphigus Vulgaris”
- Traditional corticosteroid-based therapies, while effective, are associated with significant side effects and long-term health risks. This has created a strong demand for safer and more targeted alternatives. Biologic therapies, particularly anti-CD20 monoclonal antibodies such as rituximab, have shown substantial promise by offering improved remission rates and a reduced need for steroids
- For instance, according to a 2022 article published in Frontiers in Medicine, immunotherapy has shown promise for the prevention or treatment of disease. Studies have shown the significance of T cells in pemphigus, and the function of Dsg3-specific CD4+ T cells has been elegantly demonstrated in an animal model by inducing a phenotype of interface dermatitis and PV
- Defective regulatory T (Treg) cells may also contribute to the onset of pemphigus by modulating the production of anti-Dsg3 autoantibodies. It has been shown that the main cause of humoral autoimmunity was the interaction of autoreactive T cells and B cells. As a result, the T and B immunological axis, which are implicated in the immune mechanisms of pemphigus, the primary therapeutic target for pemphigus patients
- Additionally, novel pipeline candidates like neonatal Fc receptor (FcRn) inhibitors and Bruton’s Tyrosine Kinase (BTK) inhibitors are advancing through clinical trials, aiming to further improve efficacy and patient safety. These innovative approaches are expanding the therapeutic arsenal and attracting significant investment from pharmaceutical companies
- Regulatory approvals in key markets, increasing disease awareness, and greater physician acceptance of biologics are further accelerating market penetration. As research continues, these emerging therapies are expected to reshape the treatment landscape and sustain long-term market growth
- The landscape of pemphigus vulgaris treatment is undergoing a pivotal transformation driven by biologics and precision medicine. As the demand for safer and more effective therapies grows, the Middle East and Africa market is expected to expand significantly. Innovations such as FcRn inhibitors and B-cell targeted therapies offer renewed hope for patients and new revenue streams for pharmaceutical companies
Restraint/Challenge
“Stringent Regulatory Guidelines for Pemphigus Vulgaris Drug Approval”
- Strict regulatory guidelines refer to the rigorous standards set by Middle East and Africa health authorities—such as the U.S. FDA, EMA, and others—for the development, approval, and commercialization of drugs. In the context of Pemphigus Vulgaris (PV), these regulations are crucial due to the disease’s rarity and the complexity of its treatment.
- For instance, In November 2024, an article by Oxford University Press highlighted that strict regulatory guidelines, such as those outlined by the U.S. FDA and EMA, ensure that therapies for pemphigus vulgaris undergo rigorous clinical testing to confirm their safety and efficacy. While these measures are essential for patient safety, they can delay the approval and availability of new treatments
- For instance, the FDA’s 21 CFR 312 regulations and EMA’s Committee for Medicinal Products for Human Use (CHMP) guidelines necessitate extensive phase trials before new therapies can be approved, potentially limiting patient access to promising treatments. This thorough process, while protective, can restrict treatment options in the interim
- Strict regulatory guidelines, while essential for patient safety, present both challenges and opportunities within the Middle East and Africa pemphigus vulgaris market. On one hand, they ensure that only safe and effective therapies reach patients, reinforcing confidence in approved treatments. On the other hand, lengthy approval processes and high compliance costs can delay innovation and restrict market access.
Pemphigus Vulgaris Market Scope
The market is segmented on the basis of diagnosis & treatment, population type, end-user and distribution channel.
- By Diagnosis & Treatment
On the basis of diagnosis & treatment, the market is segmented into diagnosis, treatment. In 2025, the treatment segment will dominate the market with a market share of 74.16% in 2025, driven by advancements in diagnostic technologies, increasing demand for early and accurate detection, rising awareness, and growing adoption of innovative diagnostic tools for effective management of pemphigus vulgaris.
The treatment segment is anticipated to witness the fastest growth rate of 7.6% from 2025 to 2032, fueled by technological advancements in diagnostic tools, increasing prevalence of pemphigus vulgaris, rising awareness about early detection, growing healthcare infrastructure, and expanding adoption of non-invasive and accurate diagnostic methods worldwide.
- By Population Type
On the basis of population type, the market is segmented into pediatric, adults and geriatric. The adults segment is expected to hold the largest market revenue share of 72.31% in 2025, driven by increasing diagnosis of pemphigus vulgaris in children, rising awareness among caregivers, advancements in pediatric-specific treatments, growing focus on early intervention, and enhanced healthcare access for the pediatric population in both developed and emerging regions.
The adults segment is expected to witness the fastest CAGR of 7.6% from 2025 to 2032, driven by increasing prevalence of autoimmune disorders in children, rising parental awareness, advancements in child-friendly therapies, improved diagnostic accuracy, and expanding healthcare infrastructure focused on pediatric care across both developed and emerging markets.
- By End-User
On the basis of end-user, the market is segmented into hospitals, specialty clinics, research institutes and others. The hospitals segment is expected to hold the largest market revenue share of 61.52% in 2025, driven by advanced healthcare facilities, availability of specialized medical professionals, comprehensive treatment options, higher patient footfall, growing adoption of innovative therapies, and increased government funding supporting hospital infrastructure for managing pemphigus vulgaris effectively.
The hospitals segment is expected to witness the fastest CAGR of 7.9% from 2025 to 2032, favored for its well-equipped infrastructure, availability of multidisciplinary specialists, advanced diagnostic and treatment facilities, increasing patient preference for hospital care, and expanding healthcare investments supporting better management of complex autoimmune conditions like pemphigus.
By Distribution Channel
On the basis of distribution channel, the market is segmented into hospitals pharmacy, retail pharmacy and online pharmacy. The hospitals pharmacy segment is expected to account for the largest market revenue share of 59.73% in 2025, driven by direct access to prescribed medications, strong hospital partnerships, higher patient trust, availability of specialized drugs, streamlined supply chains, and the ability to provide integrated care and counseling for patients with pemphigus vulgaris.
The hospitals pharmacy segment is expected to witness the fastest CAGR of 8.1% from 2025 to 2032, driven by increasing hospital admissions, growing demand for specialized medications, enhanced supply chain efficiency, and rising preference for in-hospital medication dispensing for pemphigus vulgaris patients.
Pemphigus Vulgaris Market Regional Analysis
- Saudi Arabia is expected to dominate the pemphigus vulgaris market with a 32.74% revenue share in 2025, supported by advanced healthcare infrastructure, high patient awareness, robust research and development activities, widespread availability of biologic therapies, and strong government initiatives promoting rare disease treatment
- Saudi Arabia’s dominance is strengthened by significant healthcare investments, a well-established pharmaceutical industry, and comprehensive insurance coverage, ensuring patient access to cutting-edge pemphigus vulgaris treatments and diagnostics
- Additionally, active collaborations between government agencies, research institutions, and biotech companies accelerate innovation and approval of novel therapies, further driving market growth in the region
Egypt Pemphigus Vulgaris Market Insight
Egypt is expected to account for the largest market revenue share in the Saudi Arabia region in 2025, attributed to advanced healthcare infrastructure, increasing awareness, rising prevalence of autoimmune disorders, and growing adoption of novel therapies. Additionally, strong government support and ongoing research boosted market growth in the region.
Pemphigus Vulgaris Market Share
The Pemphigus Vulgaris industry is primarily led by well-established companies, including:
- Sanofi (France)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- GSK plc. (U.K.)
- AstraZeneca (U.K.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Abbvie Inc. (U.S.)
- Amgen Inc (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Fresenius Kabi AG (Germany)
- GRIFOLS SA (Spain)
- Cipla Health Ltd (India)
- SUNPHARMA.NS (India)
- Zydus Group (India)
Latest Developments in Middle East and Africa Pemphigus Vulgaris Market
- In September 2021, the Phase 3 PEGASUS trial evaluated rilzabrutinib in adults with moderate-to-severe pemphigus vulgaris or foliaceus, a rare autoimmune skin disorder. Although rilzabrutinib showed a consistent safety profile with no new concerns, the study did not meet its primary or key secondary endpoints. The primary goal—complete remission between weeks 29 and 37 with low-dose corticosteroids—was not significantly different from placebo. This was the first placebo-controlled trial of a BTK inhibitor in pemphigus, highlighting the challenge of treating this complex condition.
- In February 2025, Regeneron and Sanofi submitted a supplemental Biologics License Application (sBLA) to the U.S. FDA for Dupixent to treat adults with Bullous Pemphigoid (BP), a rare autoimmune blistering disorder. This submission is based on positive data from the ADEPT Phase 2/3 trial, which showed that Dupixent significantly improved disease remission, reduced disease severity, and alleviated itch in BP patients. The FDA accepted the application for Priority Review, with a decision expected by June 20, 2025. This collaboration aims to provide a new treatment option for patients with BP, further expanding Dupixent’s role in autoimmune blistering diseases.
- In March 2025, Regeneron expanded its research into Dupixent’s potential for treating Pemphigus Vulgaris (PV), following its success in Bullous Pemphigoid (BP). Both BP and PV share underlying type 2 inflammation mechanisms, positioning Dupixent as a promising candidate for PV treatment. Ongoing studies are evaluating Dupixent’s efficacy in PV, with early results suggesting potential benefits. This exploration aims to provide a new treatment option for patients with PV, further expanding Dupixent’s application in autoimmune blistering diseases
- In April 2025, the company announced that groundbreaking of a cutting-edge biologics center of excellence in Wilmington, Delaware, spanning 470,000 square feet and representing a USD 1 billion investment.efficiency.
- In September 2024, Sanofi biologics Dupixent demonstrated significant improvements in disease remission and symptoms in both bullous pemphigoid (BP) and pemphigus vulgaris (PV), rare and life-threatening autoimmune skin diseases. In the ADEPT Phase 3 trial, five times more patients with BP on Dupixent achieved sustained remission compared to those on placebo. The study met its primary and all key secondary endpoints, showing a strong steroid-sparing effect. Dupixent is poised to become the first targeted treatment for BP and PV in the U.S. and EU, pending regulatory approval.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 PATENT ANALYSIS
4.3.1 PATIENT FLOW DIAGRAM
4.3.2 KEY PRICING STRATEGIES
4.3.3 FUTURE THERAPIES
4.3.4 KEY PATIENT ENROLLMENT STRATEGIES
4.4 MIDDLE EAST AND AFRICA CLINICAL TRIAL MARKET FOR PEMPHIGUS VULGARIS MARKET
4.5 SUPPLY CHAIN ECOSYSTEM
4.6 HEALTHCARE TARIFFS & IMPACT ON THE MARKET: MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET
4.7 EPIDEMIOLOGY: MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET
4.7.1 INCIDENCE BY GENDER
4.7.2 TREATMENT RATE
4.7.3 MORTALITY RATE
4.7.4 PATIENT TREATMENT SUCCESS RATES
5 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 EMERGING TREATMENT OPTIONS FOR PEMPHIGUS VULGARIS
6.1.2 RISING PREVALENCE OF AUTOIMMUNE DISORDERS
6.1.3 GROWING AWARENESS OF PEMPHIGUS VULGARIS
6.1.4 GROWING ADOPTION OF IMMUNOSUPPRESSIVE TREATMENTS
6.2 RESTRAINTS
6.2.1 STRINGENT REGULATORY GUIDELINES FOR PEMPHIGUS VULGARIS DRUG APPROVAL
6.2.2 HIGH TREATMENT COSTS FOR PEMPHIGUS VULGARIS THERAPIES
6.3 OPPORTUNITIES
6.3.1 INCREASING DEMAND FOR PERSONALIZED MEDICINE
6.3.2 INCREASING HEALTHCARE FACILITIES
6.3.3 RISING ADOPTION OF DIGITAL HEALTH TECHNOLOGIES FOR PEMPHIGUS VULGARIS TREATMENT MANAGEMENT
6.4 CHALLENGES
6.4.1 RISING CONCERNS OVER ADVERSE EFFECTS OF PEMPHIGUS VULGARIS MEDICATIONS
6.4.2 LACK OF REIMBURSEMENT AND COVERAGE POLICIES
7 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT
7.1 OVERVIEW
7.2 TREATMENT
7.3 DIAGNOSIS
8 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE
8.1 OVERVIEW
8.2 ADULTS
8.3 GERIATRIC
8.4 PEDIATRIC
9 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY END USER
9.1 OVERVIEW
9.2 HOSPITALS
9.3 SPECIALTY CLINICS
9.4 RESEARCH INSTITUTES
9.5 OTHERS
10 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 HOSPITALS PHARMACY
10.3 RETAIL PHARMACY
10.4 ONLINE PHARMACY
11 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY REGION
11.1 MIDDLE EAST AND AFRICA
11.1.1 SAUDI ARABIA
11.1.2 EGYPT
11.1.3 U.A.E.
11.1.4 SOUTH AFRICA
11.1.5 ISRAEL
11.1.6 BAHRAIN
11.1.7 KUWAIT
11.1.8 OMAN
11.1.9 QATAR
11.1.10 REST OF MIDDLE EAST AND AFRICA
12 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 MERCK AND CO. INC.
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENTS
14.2 SANOFI
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENT
14.3 REGENERON PHARMACEUTICALS INC.
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 PRODUCT PORTFOLIO
14.3.5 RECENT DEVELOPMENTS
14.4 JOHNSON & JOHNSON SERVICES, INC.
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENT
14.5 ASTRAZENECA
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENT
14.6 ABBVIE INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENT
14.7 ACCORD HEALTHCARE
14.7.1 COMPANY SNAPSHOT
14.7.2 PRODUCT PORTFOLIO
14.7.3 RECENT DEVELOPMENT
14.8 AMGEN INC.
14.8.1 COMPANY SNAPSHOT
14.8.2 REVENUE ANALYSIS
14.8.3 PRODUCT PORTFOLIO
14.8.4 RECENT DEVELOPMENT
14.9 ARTIVA BIOTHERAPEUTICS, INC.
14.9.1 COMPANY SNAPSHOT
14.9.2 REVENUE ANALYSIS
14.9.3 PRODUCT PIPELINE PORTFOLIO
14.9.4 RECENT DEVELOPMENT
14.1 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
14.10.1 COMPANY SNAPSHOT
14.10.2 PRODUCT PORTFOLIO
14.10.3 RECENT DEVELOPMENT
14.11 BAXTER
14.11.1 COMPANY SNAPSHOT
14.11.2 REVENUE ANALYSIS
14.11.3 PRODUCT PORTFOLIO
14.11.4 RECENT DEVELOPMENT
14.12 BIOXPRESS THERAPEUTICS SA
14.12.1 COMPANY SNAPSHOT
14.12.2 PRODUCT PORTFOLIO
14.12.3 RECENT DEVELOPMENT
14.13 CABALETTA BIO INC.
14.13.1 COMPANY SNAPSHOT
14.13.2 REVENUE ANALYSIS
14.13.3 PRODUCT PORTFOLIO
14.13.4 RECENT DEVELOPMENT
14.14 CIPLA HEALTH LTD
14.14.1 COMPANY SNAPSHOT
14.14.2 PRODUCT PORTFOLIO
14.14.3 RECENT DEVELOPMENTS
14.15 CELLTRION
14.15.1 COMPANY SNAPSHOT
14.15.2 REVENUE ANALYSIS
14.15.3 PRODUCT PORTFOLIO
14.15.4 RECENT DEVELOPMENTS
14.16 CSL
14.16.1 COMPANY SNAPSHOT
14.16.2 REVENUE ANALYSIS
14.16.3 PRODUCT PORTFOLIO
14.16.4 RECENT DEVELOPMENT
14.17 DAEWOONG PHARMACEUTICAL CO.,LTD.
14.17.1 COMPANY SNAPSHOT
14.17.2 REVENUE ANALYSIS
14.17.3 PRODUCT PORTFOLIO
14.17.4 RECENT DEVELOPMENT
14.18 F. HOFFMANN- LA ROCHE LTD.
14.18.1 COMPANY SNAPSHOT
14.18.2 REVENUE ANALYSIS
14.18.3 PRODUCT PORTFOLIO
14.18.4 RECENT DEVELOPMENT/NEWS
14.19 FRESENIUS KABI AG
14.19.1 COMPANY SNAPSHOT
14.19.2 REVENUE ANALYSIS
14.19.3 PRODUCT PORTFOLIO
14.19.4 RECENT DEVELOPMENT
14.2 GSK PLC
14.20.1 COMPANY SNAPSHOT
14.20.2 REVENUE ANALYSIS
14.20.3 PRODUCT PORTFOLIO
14.20.4 RECENT DEVELOPMENT
14.21 GRIFOLS, S.A.
14.21.1 COMPANY SNAPSHOT
14.21.2 REVENUE ANALYSIS
14.21.3 PRODUCT PORTFOLIO
14.21.4 RECENT DEVELOPMENT
14.22 INCYTE
14.22.1 COMPANY SNAPSHOT
14.22.2 REVENUE ANALYSIS
14.22.3 PRODUCT PORTFOLIO
14.22.4 RECENT DEVELOPMENT
14.23 NOVARTIS AG
14.23.1 COMPANY SNAPSHOT
14.23.2 REVENUE ANALYSIS
14.23.3 PRODUCT PORTFOLIO
14.23.4 RECENT DEVELOPMENT
14.24 LILLY
14.24.1 COMPANY SNAPSHOT
14.24.2 REVENUE ANALYSIS
14.24.3 PRODUCT PORTFOLIO
14.24.4 RECENT DEVELOPMENT
14.25 OCTAPHARMA AG
14.25.1 COMPANY SNAPSHOT
14.25.2 REVENUE ANALYSIS
14.25.3 PRODUCT PORTFOLIO
14.25.4 RECENT DEVELOPMENT/NEWS
14.26 PFIZER INC.
14.26.1 COMPANY SNAPSHOT
14.26.2 REVENUE ANALYSIS
14.26.3 PRODUCT PORTFOLIO
14.26.4 RECENT DEVELOPMENT
14.27 RAKSHIT DRUGS PVT. LTD
14.27.1 COMPANY SNAPSHOT
14.27.2 PRODUCT PORTFOLIO
14.27.3 RECENT DEVELOPMENT
14.28 SUN PHARMACEUTICALS INDUSTRIES LTD.
14.28.1 COMPANY SNAPSHOT
14.28.2 REVENUE ANALYSIS
14.28.3 PRODUCT PORTFOLIO
14.28.4 RECENT DEVELOPMENT
14.29 TEVA PHARMACEUTICAL INDUSTRIES LTD
14.29.1 COMPANY SNAPSHOT
14.29.2 REVENUE ANALYSIS
14.29.3 PRODUCT PORTFOLIO
14.29.4 RECENT DEVELOPMENT
14.3 ZYDUS GROUP
14.30.1 COMPANY SNAPSHOT
14.30.2 REVENUE ANALYSIS
14.30.3 PRODUCT PORTFOLIO
14.30.4 RECENT DEVELOPMENT
15 QUESTIONNAIRE
16 RELATED REPORTS
List of Table
TABLE 1 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, PATENT ANALYSIS
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET
TABLE 4 SURVIVAL RATES IN PEMPHIGUS VULGARIS
TABLE 5 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 6 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2028-2032 (USD MILLION)
TABLE 7 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 8 MIDDLE EAST AND AFRICA STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 9 MIDDLE EAST AND AFRICA ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 10 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 11 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 12 MIDDLE EAST AND AFRICA DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 13 MIDDLE EAST AND AFRICA DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 14 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 15 MIDDLE EAST AND AFRICA ADULTS IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 16 MIDDLE EAST AND AFRICA GERIATRIC IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 17 MIDDLE EAST AND AFRICA PEDIATRIC IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 18 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 19 MIDDLE EAST AND AFRICA HOSPITALS IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 20 MIDDLE EAST AND AFRICA SPECIALTY CLINICS IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 21 MIDDLE EAST AND AFRICA RESEARCH INSTITUTES IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 22 MIDDLE EAST AND AFRICA OTHERS IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 23 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 24 MIDDLE EAST AND AFRICA HOSPITALS PHARMACY IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 25 MIDDLE EAST AND AFRICA RETAIL PHARMACY IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 26 MIDDLE EAST AND AFRICA ONLINE PHARMACY IN PEMPHIGUS VULGARIS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 28 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 29 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 30 MIDDLE EAST AND AFRICA STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 31 MIDDLE EAST AND AFRICA ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 32 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 33 MIDDLE EAST AND AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 34 MIDDLE EAST AND AFRICA DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 35 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 36 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 37 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 38 SAUDI ARABIA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 39 SAUDI ARABIA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 40 SAUDI ARABIA STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 41 SAUDI ARABIA ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 42 SAUDI ARABIA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 43 SAUDI ARABIA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 44 SAUDI ARABIA DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 45 SAUDI ARABIA PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 46 SAUDI ARABIA PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 47 SAUDI ARABIA PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 48 EGYPT PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 49 EGYPT TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 50 EGYPT STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 51 EGYPT ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 52 EGYPT TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 53 EGYPT TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 54 EGYPT DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 55 EGYPT PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 56 EGYPT PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 57 EGYPT PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 58 U.A.E. PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 59 U.A.E. TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 60 U.A.E. STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 61 U.A.E. ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 62 U.A.E. TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 63 U.A.E. TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 64 U.A.E. DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 65 U.A.E. PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 66 U.A.E. PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 67 U.A.E. PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 68 SOUTH AFRICA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 69 SOUTH AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 70 SOUTH AFRICA STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 71 SOUTH AFRICA ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 72 SOUTH AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 73 SOUTH AFRICA TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 74 SOUTH AFRICA DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 75 SOUTH AFRICA PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 76 SOUTH AFRICA PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 77 SOUTH AFRICA PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 78 ISRAEL PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 79 ISRAEL TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 80 ISRAEL STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 81 ISRAEL ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 82 ISRAEL TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 83 ISRAEL TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 84 ISRAEL DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 85 ISRAEL PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 86 ISRAEL PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 87 ISRAEL PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 88 BAHRAIN PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 89 BAHRAIN TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 90 BAHRAIN STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 91 BAHRAIN ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 92 BAHRAIN TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 93 BAHRAIN TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 94 BAHRAIN DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 95 BAHRAIN PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 96 BAHRAIN PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 97 BAHRAIN PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 98 KUWAIT PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 99 KUWAIT TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 100 KUWAIT STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 101 KUWAIT ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 102 KUWAIT TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 103 KUWAIT TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 104 KUWAIT DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 105 KUWAIT PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 106 KUWAIT PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 107 KUWAIT PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 108 OMAN PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 109 OMAN TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 110 OMAN STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 111 OMAN ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 112 OMAN TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 113 OMAN TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 114 OMAN DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 115 OMAN PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 116 OMAN PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 117 OMAN PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 118 QATAR PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
TABLE 119 QATAR TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 120 QATAR STEROID-SPARING IMMUNOSUPPRESSANT DRUGS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 121 QATAR ANTI-INFLAMMATORY AGENTS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 122 QATAR TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 123 QATAR TREATMENT IN PEMPHIGUS VULGARIS MARKET, BY DRUG TYPE, 2018-2032 (USD MILLION)
TABLE 124 QATAR DIAGNOSIS IN PEMPHIGUS VULGARIS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 125 QATAR PEMPHIGUS VULGARIS MARKET, BY POPULATION TYPE, 2018-2032 (USD MILLION)
TABLE 126 QATAR PEMPHIGUS VULGARIS MARKET, BY END USER, 2018-2032 (USD MILLION)
TABLE 127 QATAR PEMPHIGUS VULGARIS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 128 REST OF MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD MILLION)
List of Figure
FIGURE 1 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: MIDDLE EAST AND AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET:
FIGURE 9 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: SEGMENTATION
FIGURE 11 EXECUTIVE SUMMARY
FIGURE 12 TWO SEGMENTS COMPRISE THE MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET, BY DIAGNOSIS AND TREATMENT, (2024)
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 THE EMERGING TREATMENT OPTIONS FOR THE PEMPHIGUS VULGARIS IS EXPECTED TO DRIVE THE GROWTH OF THE MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET FROM 2025 TO 2032
FIGURE 15 DIAGNOSIS AND TREATMENT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET IN 2025 AND 2032
FIGURE 16 PATIENT FLOW DESCRIPTION OF PEMPHIGUS VULGARIS:-
FIGURE 17 DROC ANALYSIS
FIGURE 18 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DIAGNOSIS AND TREATMENT, 2024
FIGURE 19 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DIAGNOSIS AND TREATMENT, 2025-2032 (USD MILLION)
FIGURE 20 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DIAGNOSIS AND TREATMENT, CAGR (2025-2032)
FIGURE 21 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DIAGNOSIS AND TREATMENT, LIFELINE CURVE
FIGURE 22 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY POPULATION TYPE, 2024
FIGURE 23 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY POPULATION TYPE, 2025-2032 (USD MILLION)
FIGURE 24 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY POPULATION TYPE, CAGR (2025-2032)
FIGURE 25 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 26 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY END USER, 2024
FIGURE 27 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY END USER, 2025-2032 (USD MILLION)
FIGURE 28 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY END USER, CAGR (2025-2032)
FIGURE 29 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 31 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD MILLION)
FIGURE 32 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 33 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: SNAPSHOT (2024)
FIGURE 35 MIDDLE EAST AND AFRICA PEMPHIGUS VULGARIS MARKET: COMPANY SHARE 2024 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.