Middle East and Africa Pharmacogenetics Testing in Psychiatry/Depression Market Analysis and Insights
Pharmacogenetic testing helps medical professionals by providing information on how a person metabolizes a medication. This information can help doctors and others avoid prescribing antidepressants that could produce undesirable outcomes. Pharmacogenomics has shown promise for predicting antidepressant response and tolerability in treating major depressive disorder (MDD). Pharmacogenomics can improve clinical outcomes by guiding antidepressant selection and dosing. The growing biotechnology sector and increasing health expenditure have accelerated the demand for pharmacogenetic testing in psychiatry/depression.
The growing prevalence of cancer disease, novel technology in the treatment of depression and or other psychiatric conditions are increasing the adoption of pharmacogenetics testing in psychiatry/depression devices and procedures, and the rising preference for non-surgical procedures are the major drivers which propelled the demand of the market in the forecast period. However, the high cost associated with the tests, stringent regulation, and lack of awareness may expect to hamper the pharmacogenetics testing in psychiatry/depression market growth in the forecast period.
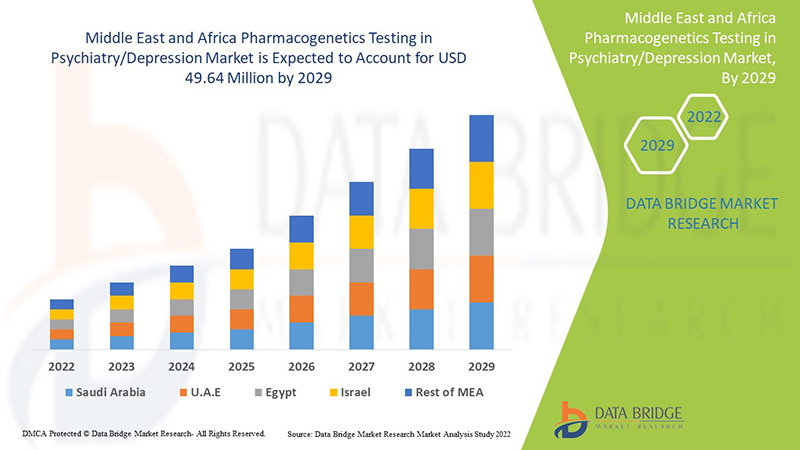
Data Bridge Market Research analyzes that the Middle East & Africa pharmacogenetics testing in psychiatry/depression market is expected to reach the value of USD 49.64 million by 2029, at a CAGR of 7.1% during the forecast period. Anxiety accounts for the largest type segment in the market due to the increasing depression rate among the Middle East & African populations. This market report also covers pricing analysis, patent analysis, and technological advancements in depth.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
The Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market, By Application (Novel Drug Candidates, Drug Optimization & Repurposing Preclinical Testing and Approval, Drug Monitoring, Finding New Diseases Associated Targets and Pathways, Understanding Disease Mechanisms, Aggregating and Synthesizing Information, Formation & Qualification of Hypotheses, De Novo Drug Design, Finding Drug Targets of an Old Drug, and Others), Technology (Machine Learning, Deep Learning, Natural Language Processing, and Others), Drug Type (Small Molecule, and Large Molecule), Offering (Software, and Services), Indication (Immuno-Oncology, Neurodegenerative Diseases, Cardiovascular Diseases, Metabolic Diseases, and Others), End Use (Contract Research Organizations (CROs), Pharmaceutical & Biotechnology Companies, Research Centers and Academic Institutes, and Others) |
|
Countries Covered |
UAE, Israel, South Africa, Egypt, Kuwait, and Rest of the Middle East, and Africa |
|
Market Players Covered |
Genelex (Part of Invitae corporation), Genewiz (Part of Azenta Life Sciences), MD Labs, BiogeneiQ, Inc., ONEOME, LLC, Myriad Genetics, Inc., GenXys, Castle Biosciences, Inc., PacBio, QIAGEN, Thermo Fisher Scientific Inc., AB-Biotics, S.A. , Coriell Life Sciences, Eurofins Scientific, Illumina, Inc., Dynamic DNA Laboratories, STADAPHARM GmbH, Color, cnsdose, Genomind, Inc., Healthspek, myDNA Life Australia Pty Ltd., HudsonAlpha, Sonic Healthcare Limited, among others. |
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Definition
Pharmacogenomic testing has recently become scalable and available to guide major depressive disorder (MDD). Clinicians increasingly recognize Pharmacogenomic (PGx) testing as an essential tool to guide medication decisions for psychiatric illnesses. Extensive implementation of PGx testing is driving the market in the forecast period.
The terms personalized medicine, stratified medicine, and precision medicine are close relatives of pharmacogenetics, but these are broader terms that also cover additional non-genetic factors. Nevertheless, pharmacogenetics is an important component of these areas. Pharmacogenetics is primarily concerned with human germline DNA variation, but there have also been important recent advances in understanding mood disorders, and mental illnesses.
Pharmacogenetics testing studies the interaction between drug and gene response of a person and searches for the gene variation which is responsible for influencing the drug effect. The test is gaining high demand as many researchers and scientists identified the unique interaction between drugs and individual genes and provides valuable insights which subsequently be used to develop customized or personalized medication.
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- INCREASE IN THE NUMBER OF PATIENTS SUFFERING FROM PSYCHIATRIC AND DEPRESSION DISORDER
Depression is a common illness worldwide, with an estimated 3.8% of the population affected, including 5.0% among adults and 5.7% among adults older than 60 years. Depression can become a serious health condition of mild to extreme severity, affecting the person to suffer greatly and can lead to suicide in the worst cases. Although over 45 antidepressants are available, suboptimal response poses a challenge and is considered a result of genetic variation, psychiatry/depression. Depending on the severity and pattern of depressive episodes over time, healthcare providers may offer psychological diagnosis such as behavioral activation, cognitive behavioral therapy, interpersonal psychotherapy, and/or antidepressant medication such as selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs). Different drugs are used for this kind of mental disorder.
With the growth in the prevalence of depression, the demand for pharmacogenetics testing is also increasing as it studies the effect of genetic variants intending to furnish tailored diagnosis. The market is expected to grow in foresting period.
- RISE IN DEMAND FOR PERSONALIZED AND PRECISION MEDICINE
Pharmacogenetics test aids the medical professional in choosing the best medicine for the person because the test searches for the gene variant that may be responsible for influencing the effect of the drug.
Medicine is beginning to get personal, and patients are gradually expressing interest in improved outcomes and less adverse effects with personalized medications. Personalized medicine has the potential to tailor the therapy with a high safety margin and the best response. This trend is largely driven by genome sequencing improvements.
The move toward personal healthcare means changes in the manufacturing of medicines. Manufacturers are moving from creating small molecules to the combination of small molecule and gene therapies. Sponsors focus on replacing inefficient large-scale batch production with investment in new technology and producing personalized drugs.
Restraint
- Lack of skilled medical and genomic expert
Most clinicians still lack confidence in pharmacogenetic (PGx) testing and subsequent data interpretation, indicating insufficient knowledge in this field. It emphasizes the need to improve literacy among healthcare professionals regarding expertise in and understanding of pharmacogenetic (PGx) testing.
Lack of practitioners awareness about the possibilities of pharmacogenetics and poor or insufficient explanation of the test results also reduce personalization technologies for patients. In addition to developing thematic training courses at medical universities, including the educational cycles in continuing professional education systems and free placement of information for practicing doctors are required: academic internet portals, webinars, etc. A clinical pharmacologist plays a crucial role in the implementation of pharmacogenetic testing.
The competence of a clinical pharmacologist in the field of pharmacogenetics is critical: he or she is the one who organizes the application of genotyping in clinical practice, interprets tests, and informs doctors about the possibilities of pharmacogenetics for patients with specific nosologies, that is, acts as the main link between the scientific world, the healthcare system, and practicing physicians in the process of introducing pharmacogenetics.
Opportunity
-
Rising advancements in technology
Advances in pharmacogenomics have introduced an increasing number of opportunities to bring personalized medicine into clinical practice for psychiatric disorders. Personalized medicine may be defined as a comprehensive, prospective approach to preventing, diagnosing, treating, and monitoring disease in ways that achieve optimal individual health care decisions. Over 100 medications now contain United States Food and Drug Administration (FDA) labelling related to potentially applicable pharmacogenomic biomarkers with technological advancements in healthcare. Also, new and advanced methods are being developed to promote pharmacogenetics testing in depression-like disorders. These tests use advanced genetic testing methods to give precise results to form a treatment regimen. The improvements in technology supporting tests improved accessibility of testing options, and the growing number of resources that help clinicians understand how to use this information when it is available are making this aspect of personalized or precision medicine a reality. Thus, providers need to become more aware of the scientific and clinical relevance of pharmacogenomic tests.
The tests also help to establish a meaningful relationship between a drug and the individual genetic makeup. This helps in deciding the drugs to be administered to the patient to treat major depressive disorders and other psychiatric conditions.
Challenge
- Strict government regulation on new products and instruments approval
The concerns regarding the efficacy and safety of products have caused most governments to develop regulatory agencies and policies to look after the development of new medical products or tests. The use of these medical products can be done after passing stringent regulatory standards, which ensure the product is safe, well studied, and has no adverse reactions.
The recent guidelines and the amendment have adequate guidance for manufacturers. International regulations such as food, drug, and administration play a major role in the new launch of the medical product or test into the market. Thus, it can be a major restraint for the market. Therefore, strict government regulation on new products and instrument approval will likely impact the market.
Post-COVID-19 Impact on the Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market
The COVID-19 outbreak had a beneficial impact on the expansion of the pharmacogenetics testing industry. The pandemic has had a negative impact on the pharmacogenomics market growth on account of the temporary halt in research activities in this field, coupled with the low influx of patients in hospitals and diagnostic centers. Since the second half of 2020, with the rising demand for research on certain drugs and testing kits for COVID-19, pharmacogenomic practices have been in vogue.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities, product launches, and strategic partnerships to improve the technology and test results involved in the pharmacogenetics testing market.
Recent Developments
- In April 2022, Blue Care Network (BCN) launched a precision medicine program, Blue Cross Personalized Medicine, which leverages pharmacogenomics, or genetic testing, to personalize and tailor medication treatments more effectively for select members based on a review of their prescribed medications for various diagnosis including behavioral health, cardiology, cardiovascular, and oncology. OneOme LLC has helped BCN achieve its precision medicine program goals, reduce the total cost of care, and improve patient health outcomes by reducing adverse drug reactions. This has helped the company to enhance its product portfolio.
- In February 2022, PacBio, a leading provider of high-quality, highly accurate sequencing platforms, announced that it is supporting The Hospital for Sick Children (SickKids) in Toronto, Canada, in using HiFi whole genome sequencing (HiFi WGS) to potentially identify genetic variants that may be associated with medical and developmental conditions. Samples that are examined using HiFi WGS were previously sequenced using short-read DNA sequencing technology but still lack the identification of a disease-causing variant. This has helped the company to enhance the use of its products.
- In July 2022, according to a new nationwide study of nearly 2,000 veterans conducted by the U.S. Department of Veterans Affairs (VA), and Major Depressive Disorder (MDD) remission rates were significantly improved when clinicians had access to GeneSight Psychotropic test results from Myriad Genetics, Inc. in largest ever mental health PGx randomized controlled trial. This has helped the company to show its progress in pharmacogenetic testing.
- In January 2021, myDNA Life Australia Pty Ltd, announced a merger with the U.S., Houston-based consumer DNA test company, FamilyTreeDNA, and its parent company. Gene by Gene for revolutionizing the field of pharmacogenomics, making truly personalized medicine a reality, before expanding into nutrigenomics to deliver actionable, personalized nutrition, fitness, and skincare recommendations. This has helped the company to expand its business.
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Scope
Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into type, test type, gene type, patient type, product, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Type
- Anxiety
- Mood Disorders
- Depression
- Bipolar Disorders
- Psychotic Disorders
- Eating Disorders
On the basis of type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into anxiety, mood disorders, depression, bipolar disorders, psychotic disorders, and eating disorders.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Test Type
- Whole Genome Sequencing
- Chromosomal Array-Based Tests
On the basis of test type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into whole genome sequencing, and chromosomal array-based tests.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Gene Type
- CYP2C19
- CYP2C9 AND VKORC1
- CYP2D6
- HLA-B
- HTR2A/C
- HLA-A
- CYP3A4
- SLC6A4
- MTHFR
- COMT
- OTHERS
On the basis of gene type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT, and others.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Patient Type
- Child
- Adult
- Geriatric
On the basis of patient type, the Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into child, adult, and geriatric.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Product
- Instruments
- Consumables
- Software & Services
On the basis of product type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into instruments, consumables, and software & services.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By End User
- Hospitals & Clinics
- Dignostics Laboratories
- Academic And Research Institutes
- Others
On the basis of end users, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into hospitals and clinics, diagnostics laboratories, academic and research institutes, and others.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Distribution Channel
- Direct Tender
- Third-Party Distribution
- Hospital Pharmacy
- Others

On the basis of distribution channel, the Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into direct tender, third party distribution hospital pharmacy, and others.
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Regional Analysis/Insights
The Middle East & Africa pharmacogenetics testing in psychiatry/depression market is analyzed, and market size information is provided by the type, test type, gene type, patient type, product, end user, and distribution channel. The countries covered in this market report are UAE, Israel, South Africa, Egypt, Kuwait, and rest of the Middle East & Africa.
In 2022, Middle East & Africa is dominating due to the presence of key market players in the largest consumer market with high GDP. South Africa is expected to grow due to the rise in technological advancement in pharmacogenetics testing.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Middle East & African brands, the challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Share Analysis
Middle East & Africa pharmacogenetics testing in psychiatry/depression market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Middle East & Africa pharmacogenetics testing in psychiatry/depression market.
Some of the major players operating in the Middle East & Africa pharmacogenetics testing in psychiatry/depression market are
- Genelex (Part of Invitae corporation)
- Genewiz (Part of Azenta Life Sciences)
- MD Labs
- BiogeneiQ, Inc.
- ONEOME, LLC
- Myriad Genetics, Inc.
- GenXys
- Castle Biosciences, Inc.
- PacBio
- QIAGEN
- Thermo Fisher Scientific Inc.
- AB-Biotics.S.A.
- Coriell Life Sciences
- Eurofins Scientific
- Illumina, Inc.
- Dynamic DNA Laboratories
- STADAPHARM GmbH
- Color
- Cnsdose
- Genomind, Inc.
- Healthspek
- myDNA Life Australia Pty Ltd.
- HudsonAlpha
- Sonic Healthcare Limited
Research Methodology: Middle East & Africa pharmacogenetics testing in psychiatry/depression market
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Middle East & Africa vs Regional, and Vendor Share Analysis. Please request an analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PESTEL ANALYSIS
3.2 PORTER'S FIVE FORCES MODEL
3.3 INDUSTRIAL INSIGHTS:
3.4 PIPELINE ANALYSIS FOR MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
3.5 EPIDEMIOLOGY
4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, REGULATIONS
4.1 UNITED STATES (U.S.)
4.1.1 ROLE OF FDA
4.1.2 ROLE OF CDC AND HCFA
4.2 EUROPEAN UNION (EU)
4.3 FRANCE
4.4 AUSTRALIA
4.5 SOUTH KOREA
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 INCREASE IN THE NUMBER OF PATIENTS SUFFERING FROM PSYCHIATRIC AND DEPRESSION DISORDER
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 RISE IN DEMAND FOR PERSONALIZED AND PRECISION MEDICINE
5.2 RESTRAINTS
5.2.1 LACK OF SKILLED MEDICAL AND GENOMIC EXPERT
5.2.2 LACK OF STRONG CLINICAL EVIDENCE
5.2.3 HIGH COST ASSOCIATED WITH THE DIAGNOSIS
5.3 OPPORTUNITIES
5.3.1 RISING ADVANCEMENTS IN TECHNOLOGY
5.3.2 INCREASING NUMBER OF KEY PLAYERS IN MARKET
5.4 CHALLENGES
5.4.1 STRICT GOVERNMENT REGULATION ON NEW PRODUCTS AND INSTRUMENTS APPROVAL
5.4.2 DEARTH OF SKILLED PERSONNEL
6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
6.1 OVERVIEW
6.2 ANXIETY
6.3 DEPRESSION
6.4 MOOD DISORDERS
6.5 BIPOLAR DISORDERS
6.6 EATING DISORDERS
6.7 PSYCHOTIC DISORDERS
7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT
7.1 OVERVIEW
7.2 CONSUMABLES
7.2.1 WHOLE GENOME SEQUENCING KITS
7.2.2 CHROMOSOMAL ARRAY BASED KITS
7.3 INSTRUMENTS
7.4 SOFTWARE AND SERVICES
8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 WHOLE GENOME SEQUENCING
8.2.1 EXOME SEQUENCING
8.2.1.1 SNP
8.2.1.2 CNV
8.2.1.3 RARE PTV MUTATION
8.2.1.4 ULTRA PTV MUTATION
8.2.1.5 OTHERS
8.2.2 KARYOTYPE
8.2.2.1 SNP
8.2.2.2 CNV
8.2.2.3 RARE PTV MUTATION
8.2.2.4 ULTRA PTV MUTATION
8.2.2.5 OTHERS
8.2.3 LOW COVERAGE WGS
8.2.3.1 SNP
8.2.3.2 CNV
8.2.3.3 RARE PTV MUTATION
8.2.3.4 ULTRA PTV MUTATION
8.2.3.5 OTHERS
8.2.4 DEEP COVERAGE WGS
8.2.4.1 SNP
8.2.4.2 CNV
8.2.4.3 RARE PTV MUTATION
8.2.4.4 ULTRA PTV MUTATION
8.2.4.5 OTHERS
8.2.5 MICROARRAY
8.2.5.1 SNP
8.2.5.2 CNV
8.2.5.3 RARE PTV MUTATION
8.2.5.4 ULTRA PTV MUTATION
8.2.5.5 OTHERS
8.2.6 OTHERS
8.3 CHROMOSOMAL ARRAY BASED TESTS
8.3.1 MICRODELETIONS
8.3.2 MICRO DUPLICATIONS
9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE
9.1 OVERVIEW
9.2 CYP2C19
9.3 CYP2C9 AND VKORC1
9.4 CYP2D6
9.5 HLA-B
9.6 HTR2A/C
9.7 HLA-A
9.8 CYP3A4
9.9 SLC6A4
9.1 MTHFR
9.11 COMT
9.12 OTHERS
10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 ADULT
10.3 GERIATRIC
10.4 CHILD
11 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET , BY END USER
11.1 OVERVIEW
11.2 HOSPITAL AND CLINICS
11.2.1 DRUG EFFECTIVENESS
11.2.2 SIDE EFFECTS
11.2.3 DOSAGE
11.3 DIAGNOSTICS LABORATORIES
11.3.1 DRUG EFFECTIVENESS
11.3.2 SIDE EFFECTS
11.3.3 DOSAGE
11.4 ACADEMIC AND RESEARCH INSTITUTES
11.4.1 DRUG EFFECTIVENESS
11.4.2 SIDE EFFECTS
11.4.3 DOSAGE
11.5 OTHERS
12 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 THIRD PARTY DISTRIBUTION
12.4 HOSPITAL PHARMACY
12.5 OTHERS
13 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SOUTH AFRICA
13.1.2 SAUDI ARABIA
13.1.3 U.A.E
13.1.4 ISRAEL
13.1.5 EGYPT
13.1.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 THERMO FISHER SCIENTIFIC INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 RECENT FINANCIALS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 ILLUMINA, INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 MYRIAD GENETICS, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 SONIC HEALTHCARE LIMITED
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 QIAGEN
16.5.1 COMPANY SNAPSHOT
16.5.2 RECENT FINANCIALS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 EUROFINS SCIENTIFIC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AB-BIOTICS, S.A.
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 BIOGENIQ INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 CASTLE BIOSCIENCE, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CNSDOSE
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 COLOR HEALTH, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CORIELL LIFE SCIENCES
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENTS
16.13 DYNAMIC DNA LABORATORIES
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 GENELEX (SUBSIADIARY OF INVITAE CORPORATION.)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 GENEWIZ (PART OF AZENTA LIFE SCIENCES)
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GENOMIND, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 GENXYS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 HEALTHSPEK
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 HUDSONALPHA
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 MD LABS
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 MYDNA LIFE AUSTRALIA PTY LTD.
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 ONEOME, LLC
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENTS
16.23 PACBIO
16.23.1 COMPANY SNAPSHOT
16.23.2 REVENUE ANALYSIS
16.23.3 PRODUCT PORTFOLIO
16.23.4 RECENT DEVELOPMENTS
16.24 STADAPHARM GMBH
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA ANXIETY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA DEPRESSION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA MOOD DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA BIPOLAR DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA EATING DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA PSYCHOTIC DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 10 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 13 MIDDLE EAST & AFRICA INSTRUMENTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA SOFTWARE AND SERVICES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA CYP2C19 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA CYP2C9 AND VKORC1 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA CYP2D6 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA HLA-B IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA HTR2A/C IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HLA-A IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA CYP3A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA SLC6A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA MTHFR IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA COMT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA ADULT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA GERIATRIC IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA CHILD IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA DIRECT TENDER IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA THIRD PARTY DISTRIBUTION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA HOSPITAL PHARMACY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 54 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 55 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 57 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 58 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 59 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 60 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 61 MIDDLE EAST AND AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 62 MIDDLE EAST AND AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 63 MIDDLE EAST AND AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 64 MIDDLE EAST AND AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 65 MIDDLE EAST AND AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 66 MIDDLE EAST AND AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 67 MIDDLE EAST AND AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 69 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 70 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 MIDDLE EAST AND AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 72 MIDDLE EAST AND AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 73 MIDDLE EAST AND AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 77 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 78 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 79 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 81 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 82 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 83 SOUTH AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 84 SOUTH AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 85 SOUTH AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 86 SOUTH AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 87 SOUTH AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 88 SOUTH AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 89 SOUTH AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 91 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 92 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 93 SOUTH AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 SOUTH AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 SOUTH AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 96 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 99 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 100 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 101 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 103 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 104 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 105 SAUDI ARABIA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 106 SAUDI ARABIA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 107 SAUDI ARABIA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 108 SAUDI ARABIA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 109 SAUDI ARABIA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 110 SAUDI ARABIA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 111 SAUDI ARABIA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 113 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 114 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 115 SAUDI ARABIA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 116 SAUDI ARABIA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 117 SAUDI ARABIA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 118 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 119 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 120 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 122 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 123 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 124 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 125 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 126 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 127 UAE WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 128 UAE EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 129 UAE KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 130 UAE LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 131 UAE DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 132 UAE MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 133 UAE CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 135 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 136 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 137 UAE HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 138 UAE DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 139 UAE ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 140 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 141 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 143 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 144 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 145 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 146 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 147 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 148 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 149 ISRAEL WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 150 ISRAEL EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 151 ISRAEL KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 152 ISRAEL LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 153 ISRAEL DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 154 ISRAEL MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 155 ISRAEL CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 157 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 158 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 159 ISRAEL HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 160 ISRAEL DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 161 ISRAEL ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 162 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 163 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 164 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 165 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 166 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 167 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 168 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 169 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 170 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 171 EGYPT WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 172 EGYPT EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 173 EGYPT KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 174 EGYPT LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 175 EGYPT DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 176 EGYPT MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 177 EGYPT CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 179 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 180 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 181 EGYPT HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 182 EGYPT DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 183 EGYPT ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 184 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 185 REST OF MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 11 THE INCREASING ADOPTION OF PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION DEVICES AND PROCEDURES AND RISING PREFERENCE FOR NON-SURGICAL PROCEDURES ARE EXPECTED TO DRIVE THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SOFTWARE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN THE PSYCHIATRY/DEPRESSION MARKET
FIGURE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021
FIGURE 16 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 17 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 18 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 19 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2021
FIGURE 20 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 21 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 22 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 23 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2021
FIGURE 24 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 26 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 27 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2021
FIGURE 28 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2022-2029 (USD MILLION)
FIGURE 29 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, CAGR (2022-2029)
FIGURE 30 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, LIFELINE CURVE
FIGURE 31 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2021
FIGURE 32 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 33 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 34 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 35 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2021
FIGURE 36 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, CAGR (2022-2029)
FIGURE 38 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, LIFELINE CURVE
FIGURE 39 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2021)
FIGURE 44 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021)
FIGURE 45 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE (2022-2029)
FIGURE 48 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












