North America Adalimumab Market
Market Size in USD million
CAGR :
% 
 USD
16,879.80 million
USD
25,709.40 million
2022
2030
USD
16,879.80 million
USD
25,709.40 million
2022
2030
| 2023 –2030 | |
| USD 16,879.80 million | |
| USD 25,709.40 million | |
|
|
|
North America Adalimumab Market Analysis and Size
Numerous research studies are taking place which is projected to create a cutting edge advantage for manufacturers to launch new and innovative adalimumab which will provide numerous other opportunities in the market. The market for biopharmaceuticals has grown majorly than the overall pharmaceutical market in recent years because of the enormous demand for these medications, and is thought to have a major amount of potential for dynamic growth in future.
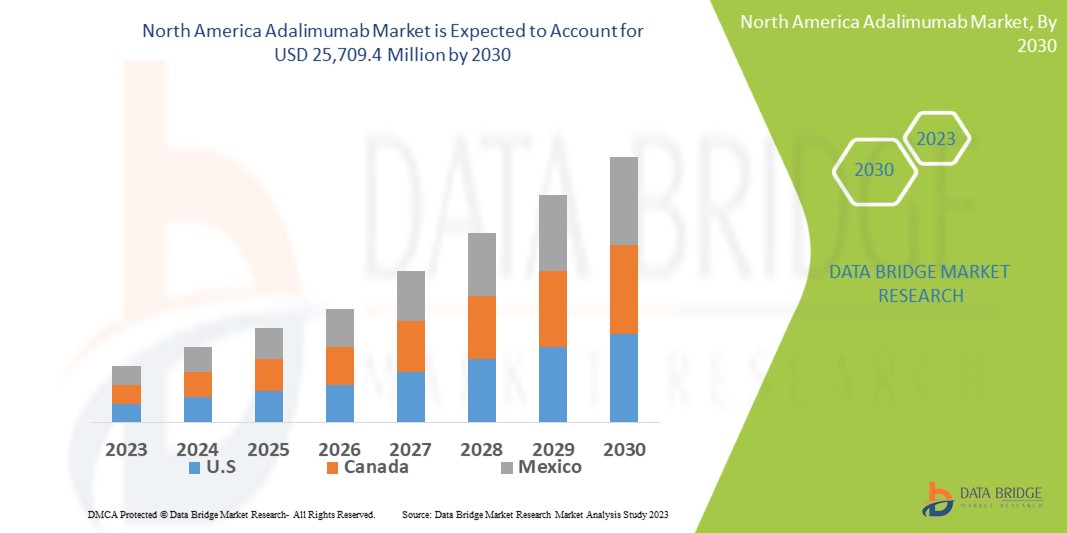
Data Bridge Market Research analyses a growth rate in the adalimumab market in the forecast period 2023-2030. The expected CAGR of adalimumab market is tend to be around 5.40 % in the mentioned forecast period. The market was valued at USD 16,879.8 million in 2022, and it would grow upto USD 25,709.4 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
North America Adalimumab Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Indication (Rheumatoid Arthritis, Ankylosing Spondylitis, Chronic Plaque Psoriasis, Crohn's Disease, Ulcerative Colitis, Psoriatic Arthritis, Juvenile Idiopathic Arthritis, Hidradenitis Suppurativa, Non-Infectious Intermediate, Others), Type (Biologics, Biosimilars), Dosage Strength (40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.2mlg, 10mg/0.1mlg, Others), Drug Type (Branded, Generics), Route of Administration (Parenteral, Oral), Population Type (Adults, Children), End User (Hospital/Clinical Laboratories, Physician Offices, Reference Laboratories and Other End Users), Distribution Channel (Direct Tender, Retail Sales and Other) |
|
Countries Covered |
U.S., Canada and Mexico in North America |
|
Market Players Covered |
Innovent Biologics, Inc. (China), Hetero Biopharma Ltd. (India), Reliance Life Sciences (India), F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Sanofi (France), Pfizer Inc. (U.S.), GSK plc (U.K.), Novartis AG (Switzerland), Zydus Group (India), Biogen (U.S.), Fresenius Kabi AG (Germany), Boehringer Ingelheim International GmbH. (Germany), Amgen Inc. (U.S.), AbbVie Inc. (U.S.), Abbott (U.S.) |
|
Market Opportunities |
|
Market Definition
Adalimumab is one of the monoclonal antibodies widely used to treat some autoimmune diseases involving rheumatoid arthritis and crohn's diseases among others. Adalimumab is an anti- TNF drug specifically indicated for treating several inflammatory symptoms. This drug works by binding to the TNF factor-alpha, decreasing the increasing chances of the inflammatory response to some autoimmune diseases.
North America Adalimumab Market Dynamics
Drivers
- Increased Geriatric Population
The increasing elderly population will boost the market’s growth rate during the forecast period of 2023-2030According to the World Ageing 2020 research, the population will be around 727 million people aged 65 years and up in 2020 globally. In the year 2050, the number of old people is anticipated to rise upto 1.5 billion. It has been closely witnessed that the elderly population is more vulnerable to chronic ailments, which is expected to boost the market’s growth rate.
- Rising Incidence of Autoimmune Disorders
The increase in prevalences of autoimmune disease such as plaque psoriasis, rheumatoid arthritis ulcerative colitis, psoriatic arthritis, ankylosing spondylitis, and Crohn's disease is expected to enhance the growth rate of the market. As per an article titled “The Prevalence and Statistics of Arthritis” published in 2022, the occurrence of rheumatoid arthritis is 2 to 3 times higher in women compared to men. In both men and women, the initiation of rheumatoid arthritis is more among people in their 60s. Additionally, the growing prevalence of chronic disorders will rise the demand of adalimumab market.
Opportunities
- Increasing Drug Launches
Growing launches of novel biosimilar and increasing penetration of generic drugs are projected to boost the market. For instance, Dr. Reddy's Laboratories launched a generic drug in February 2022. Moreover, Biocon stated strong growth in its biosimilars business and the launch of the first interchangeable biosimilar glargine in the U.S. in January 2022. Furthermore, ABP 501, a novel Adalimumab biosimilar by Amgen, is still waiting for approval. Lately approved biologics such as Hyrimoz by Novartis and ABRILADA by Pfizer are set to have strong market penetration because of a favourable reimbursement scenario.
- Patient Awareness Towards Arthritis
Increasing patient awareness associated with arthritis disorders and the influx of new biopharmaceuticals in the global time are expected to surge the adoption of adalimumab drugs. For instance, as per the article titled “The Prevalence and Statistics of Arthritis” published in February 2022, the incidence of arthritis has increased, and it will continue to rise with the passing decade. This factor boosts the growth of the market.
Restraints/Challenges
- Side Effects of Adalimumab Drugs
The side-effects of this drug such as nausea, vomiting, internal bleeding, anaphylaxis, and respiratory tract infections may limit the market's growth.
- High Cost of Treatment
The increasing expenditure which is required for developing these techniques limit the market growth. Several market players make huge investment in manufacturing new and advanced machines to faster the process and in return the cost is increased. This factor limits the market growth.
This adalimumab market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the adalimumab market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In 2021, The U.S. FDA announced the approval of the first interchangeable biosimilar product for treating numerous inflammatory diseases. The biosimilar and interchangeable approval pathway was set to help patients with critical medical conditions gain access to more treatment options. It is said that Cyltezo is the first interchangeable monoclonal antibody and the second interchangeable biosimilar medicine authorized by the FDA.
North America Adalimumab Market
The adalimumab market is segmented on the basis indication, type, dosage strength, drug class, route of administration, population type, end user and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug class
- Antirheumatics
- TNF Alfa Inhibitors
- Others
Indication
- Rheumatoid Arthritis
- Ankylosing Spondylitis
- Chronic Plaque Psoriasis
- Crohn's Disease
- Ulcerative Colitis
- Psoriatic Arthritis
- Juvenile Idiopathic Arthritis
- Hidradenitis Suppurativa
- Non-Infectious Intermediate
- Others
Type
- Biologics
- Biosimilars
Dosage Strength
- 40mg/0.4mlg
- 80mg/0.8mlg
- 20mg/0.2mlg
- 10mg/0.1mlg
- Others
Route of Administration
- Oral
- Parenteral
- Others
Population Type
- Injection
- Solution
- Tablet
- Others
Age Group
- Pediatric
- Adult
- Geriatric
End User
- Hospital/Clinical Laboratories
- Physician Offices
- Reference Laboratories
- Others
Distribution Channel
- Direct Tender
- Retail Sales
- Other
North America Adalimumab Market Regional Analysis/Insights
The adalimumab market is analyzed and market size insights and trends are provided by indication, type, dosage strength, drug class, route of administration, population type, end user and distribution channel as referenced above.
The major countries covered in the adalimumab market report are the U.S., Canada and Mexico in North America.
The U.S. is leading the market in North America as Humira (Adalimumab) is one of the common and widely known drugs in the market. Favourable regulatory policies for biosimilars are projected to boost the demand for adalimumab during the forecast period.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Adalimumab Market Share Analysis
The adalimumab market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to adalimumab market
Key players operating in the adalimumab market include:
- Innovent Biologics, Inc. (China)
- Hetero Biopharma Ltd. (India)
- Reliance Life Sciences (India)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Zydus Group (India)
- Biogen (U.S.)
- Fresenius Kabi AG (Germany)
- Boehringer Ingelheim International GmbH. (Germany)
- Amgen Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Abbott (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.