North America Antiviral Drugs Market Analysis and Insights
The increasing awareness about viral infections North America has enhanced the demand for the market. The rising healthcare expenditure for better health services also contributes to the market's growth. The major market players focus on various service launches and approvals during this crucial period. In addition, the increase in improved advancement of drug development techniques also contributes to the rising demand for antiviral drugs.
The North America antiviral drugs market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in the developmental activity for launching novel services in the market. The increasing development in the field of drug development is further boosting the market growth. However, difficulties such as the lack of standardized protocols and the lack of skilled professionals might hamper the growth of the North America antiviral drugs market in the forecast period.
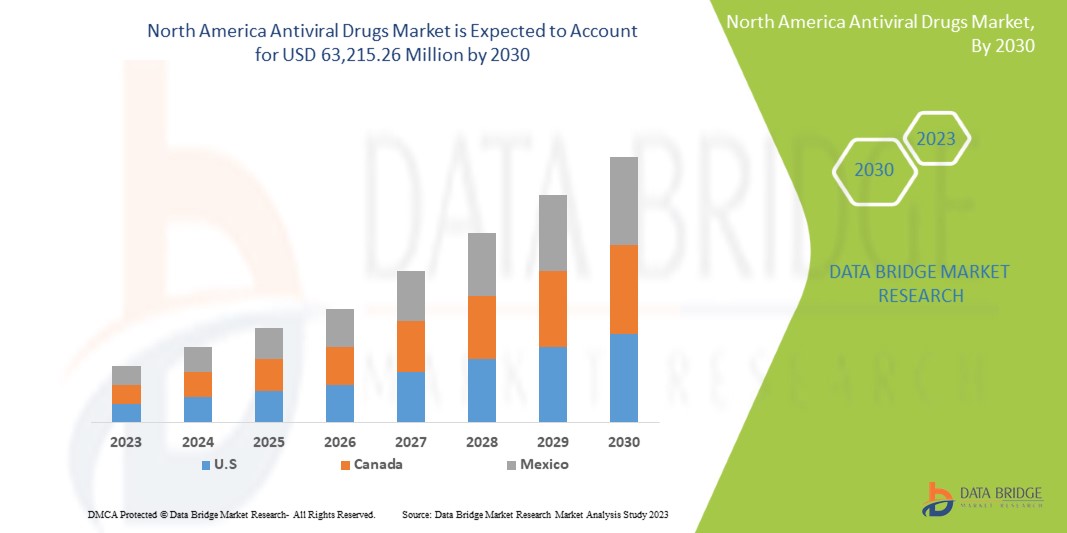
Increasing healthcare expenditure on advancement and drug development is expected to give opportunities to the market. However, the increasing use of alternative treatments may challenge market growth. Data Bridge Market Research analyzes that the North America antiviral drugs market is expected to reach the value of USD 63,215.26 million by 2030, with a CAGR of 5.5% during the forecast period.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
Indication (Influenza, Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), Respiratory Syncytial Virus, Herpes Simplex Virus, Human Cytomegalovirus (HCMV), Varicella-Zoster Virus (VZV), Hepatitis B Virus (HBV), Coronavirus Infection, and Others), Patient Type (Child, Adult, and Geriatric), Products (Oral, Topical, and Parenteral), Drug Type (Generic and Branded), End User (Hospitals, Clinics, Home Healthcare, Specialty Centers, Ambulatory Centers, and Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy’s Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc, and Hetero. among others |
North America Antiviral Drugs Market Definition
Antiviral drugs are medications used to treat viral infections by inhibiting the replication of viruses within host cells. These drugs target specific viruses or types of viruses and work by either preventing the virus from entering the host cell or by blocking key enzymes or proteins required for viral replication. Unlike antibiotics, which are used to treat bacterial infections, antiviral drugs are generally less effective, as viruses have a much simpler structure and rely on host cells to replicate. However, they can still be useful in treating some viral infections, such as influenza, herpes, and HIV.
North America Antiviral Drugs Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Rising prevalence of viral infections
Viral infections have steadily increased around the world during the past few decades. A virus that enters the body, exploits its cells for replication and spreads causes a viral infection. Viral infections can result in various symptoms, from minor to major, and in some instances, even life-threatening ones. Globalization is one of the main causes of the increase in viral infections. People travel and communicate with each other across boundaries, making the world more connected than ever. Viral transmission from one area to another has accelerated due to this enhanced connectedness.
Thus, the rising prevalence of viral infections is a complex issue with many contributing factors. Globalization, population density, climate change, and antibiotic resistance all influence the spread of viruses. Hence, it is expected to drive market growth.
- Advancements in new antiviral drug development
Antiviral treatments are prescribed to patients with viral infections. The creation of novel antiviral medications has made tremendous strides throughout time. These developments have lowered the disease burden, improved the treatment of viral infections, and saved lives. There is much development in the field of new antiviral drugs.
Thus, advancements in new antiviral drug development have improved the treatment of viral infections, reduced the disease burden, and are expected to drive market growth.
Restraint
- High cost of antiviral drugs
The high cost of antiviral drugs can have significant implications for patients and healthcare systems. Patients who cannot afford these medications may go without treatment or rely on inferior treatments, leading to worse health outcomes. In addition, the high cost of antiviral drugs can strain healthcare budgets, particularly in countries with limited resources.
Thus, the high cost of antiviral drugs is expected to restrain the North America antiviral drugs market.
Opportunity
-
Rising novel drug delivery systems
Research on antiviral medications has emphasized creating novel drug delivery mechanisms. Over conventional drug administration techniques, novel delivery systems have several benefits, such as improved bioavailability, tailored drug delivery, and fewer adverse effects.
Thus, developing novel drug delivery systems is an important area of research in antiviral drugs. Nanoparticle drug delivery systems, hydrogels, dendrimers, microneedles, and cell-penetrating peptides are some promising drug delivery systems that have been investigated for antiviral drugs. These delivery systems offer several advantages over traditional drug delivery methods and have the potential to improve the efficacy and safety of antiviral drugs and are expected to create an opportunity in the market's growth.
Challenge
- Patent expiration of antiviral drugs
The process of patent expiration results in the original developer or patent holder losing their exclusive right to produce and market a specific medicine. The patent expiration of antiviral medications may significantly impact the pharmaceutical business since it may invite competition from producers of generic medications.
Antiviral medications' patents expire at the end of the time in which their creator has the sole right to manufacture and market the medication. Following the expiration of a drug's patent, other producers can create and market generic versions. This may result in greater competition and lower consumer prices. HIV, hepatitis B and C, herpes, influenza, and other viral illnesses are all treated with antiviral medications. Antiviral medications' patents expire at different times depending on the drug and the nation. Drug patents are typically granted for 20 years from the date of filing. Other producers are free to create and market generic versions of medicine once its patent has expired. Because the producer does not have to spend as much on marketing, research and development, and clinical studies, generic medications are often more affordable than name-brand medications.
Thus, patent expiration of antiviral drugs can have significant implications for the availability, affordability, and accessibility of these important medications and is expected to act as a challenge to market growth.
Recent Developments
- In January 2023, Merck, known as MSD, announced the successful completion of the cash tender offer, through a subsidiary, for all of the outstanding shares of common stock of Imago Biosciences, Inc. (Nasdaq: IMGO), at a purchase price of USD 36.00 per share in cash, without interest and subject to deduction for any required tax withholding. The acquisition will help in the growth of the revenue.
- In April 2021, Zydus Pharmaceuticals, Inc. announced that it had received restricted emergency use approval from the Drug Controller General of India (DCGI) to use the antiviral drug Virafin for the treatment of moderate COVID-19 infections. This will help the company to increase its North America presence and reputation in other regions of the globe.
North America Antiviral Drugs Market Scope
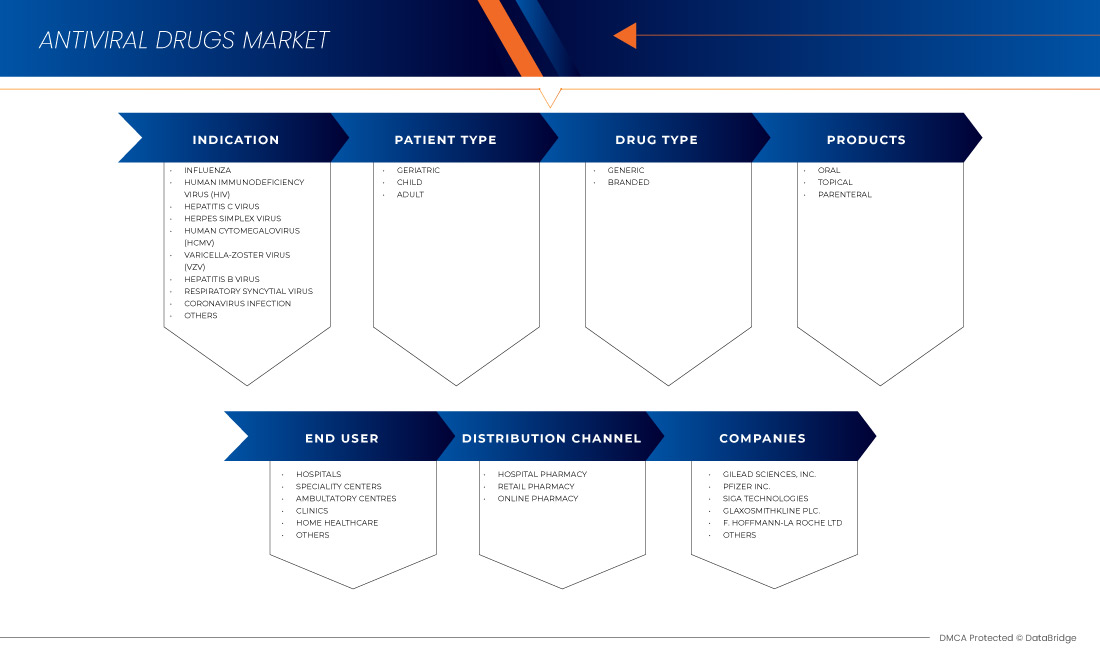
The North America antiviral drugs market is segmented into six notable segments based on indication, patient type, products, drug type, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Indication
- Influenza
- Human Immunodeficiency Virus (HIV)
- Hepatitis C Virus
- Herpes Simplex Virus
- Human Cytomegalovirus (HCMV)
- Varicella-Zoster Virus (VZV)
- Hepatitis B Virus
- Respiratory Syncytial Virus
- Coronavirus Infection
- Others
On the basis of indication, the North America antiviral drugs market is segmented into influenza, human immunodeficiency virus (HIV), hepatitis C virus (HCV), respiratory syncytial virus, herpes simplex virus, human cytomegalovirus (HCMV), varicella-zoster virus (VZV), hepatitis B virus (HBV), coronavirus infection, and others.
Patient Type
- Child
- Adult
- Geriatric
On the basis of patient type, the North America antiviral drugs market is segmented into child, adult, and geriatric.
PRODUCTS
- Oral
- Topical
- Parenteral
On the basis of products, the North America antiviral drugs market is segmented into oral, topical, and parenteral.
Drug Type
- Generic
- Branded
On the basis of drug type, the North America antiviral drugs market is segmented into generic and branded.
End User
- Hospital
- Clinics
- Home Healthcare
- Speciality Centers
- Ambulatory Centers
- Others
On the basis of end user, the North America antiviral drugs market is segmented into hospitals, clinics, home healthcare, specialty centers, ambulatory centers, and others.
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
On the basis of distribution channel, the North America antiviral drugs market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy.
North America Antiviral Drugs Market Regional Analysis/Insights
The North America antiviral drugs market is categorized into six notable segments based on indication, patient type, products, drug type, end user, and distribution channel.
The countries covered in this market report U.S., Canada, and Mexico.
In 2023, U.S., dominates the North America region due to the strong presence of key players and due to the increasing demand from emerging markets and expansion
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Antiviral Drugs Market Share Analysis
North America antiviral drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product approvals, product width and breath, application dominance, and product type lifeline curve. The above data points provided are only related to the company’s focus on the North America antiviral drugs market.
Some of the major players operating in the North America antiviral drugs market are Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy’s Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc, and Hetero. among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR NORTH AMERICA ANTIVIRAL DRUGS MARKET
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING PREVALENCE OF VIRAL INFECTIONS
8.1.2 ADVANCEMENTS IN NEW ANTIVIRAL DRUG DEVELOPMENT
8.1.3 GROWING DEMAND FOR COMBINATION THERAPIES
8.1.4 INCREASING GOVERNMENT FUNDING AND R&D ACTIVITIES
8.2 RESTRAINS
8.2.1 HIGH COST OF ANTIVIRAL DRUGS
8.2.2 EMERGENCE OF DRUG-RESISTANT STRAINS OF VIRUSES
8.3 OPPORTUNITIES
8.3.1 INCREASING COLLABORATION AND PARTNERSHIP AMONG KEY PLAYERS
8.3.2 RISING NOVEL DRUG DELIVERY SYSTEMS
8.3.3 DEVELOPMENT OF PERSONALIZED MEDICINES
8.4 CHALLENGES
8.4.1 PATENT EXPIRATION OF ANTIVIRAL DRUGS
8.4.2 DEMAND FOR ALTERNATIVE MEDICINES
9 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION
9.1 OVERVIEW
9.2 INFLUENZA
9.2.1 NEURAMINIDASE INHIBITORS
9.2.1.1 OSELTAMIVIR
9.2.1.2 ZANAMIVIR
9.2.1.3 PERAMIVIR
9.2.1.4 LANINAMIVIR
9.2.2 M2 INHIBITORS
9.2.2.1 RIMANTADINE
9.2.2.2 OTHERS
9.2.3 RNA POLYMERASE INHIBITORS
9.2.3.1 FAVIPIRAVIR
9.2.3.2 BALOXAVIR MARBOXIL
9.3 HUMAN IMMUNODEFICIENCY VIRUS (HIV)
9.3.1 REVERSE TRANSCRIPTASE INHIBITORS
9.3.1.1 NUCLEOSIDE (NRTIS)
9.3.1.1.1 LAMIVUDINE
9.3.1.1.2 ABACAVIR
9.3.1.1.3 DIDANOSINE
9.3.1.1.4 OTHERS
9.3.1.2 NONNUCLEOSIDE (NNRTIS)
9.3.1.2.1 EFAVIRENZ
9.3.1.2.2 NEVIRAPINE
9.3.1.2.3 DELAVIRDINE
9.3.1.2.4 OTHERS
9.3.1.3 INTEGRASE
9.3.1.3.1 DOLUTEGRAVIR
9.3.1.3.2 ELVITEGRAVIR
9.3.1.3.3 RALTEGRAVIR
9.3.1.3.4 BICTEGRAVIR
9.3.1.4 NUCLEOTIDE
9.3.1.4.1 TENOFOVIR
9.3.1.4.2 OTHERS
9.3.1.5 INTERFERONS
9.3.1.5.1 ALPHA
9.3.1.5.2 BETA
9.3.1.5.3 GAMMA
9.3.1.6 GP41
9.3.1.6.1 ENFUVIRTIDE
9.3.1.6.2 OTHERS
9.3.2 PROTEASE
9.3.2.1 ATAZANAVIR
9.3.2.2 DARUNAVIR
9.3.2.3 LOPINAVIR
9.3.2.4 RITONAVIR
9.3.2.5 SAQUINAVIR
9.3.2.6 INDINAVIR
9.3.2.7 NELFINAVIR
9.3.2.8 TIPRANAVIR
9.3.2.9 AMPRENAVIR
9.4 HEPATITIS C VIRUS
9.4.1 NS5B POLYMERASE
9.4.1.1 SOFOSBUVIR
9.4.1.2 DASABUVIR
9.4.2 NS3/4A PROTEASE
9.4.2.1 DANOPREVIR
9.4.2.2 GLECAPREVIR
9.4.2.3 GRAZOPREVIR
9.4.2.4 PARITAPREVIR
9.4.2.5 SIMEPREVIR
9.4.3 NS5A PHOSPHOPROTEIN
9.4.3.1 LEDIPASVIR
9.4.3.2 VELPATASVIR
9.4.3.3 OMBITASVIR
9.4.3.4 ELBASVIR
9.4.3.5 DACLATASVIR
9.4.3.6 PIBRENTASVIR
9.4.4 NEURAMINIDASE
9.4.4.1 OSELTAMIVIR
9.4.4.2 ZANAMIVIR
9.4.4.3 PERAMIVIR
9.4.4.4 LANINAMIVIR
9.4.5 RNA POLYMERASE
9.4.5.1 BALOXAVIR MARBOXIL
9.4.5.2 FAVIPIRAVIR
9.4.6 MATRIX PROTEIN 2
9.4.6.1 RIMATIDINE
9.4.6.2 FAVIPIRAVIR
9.5 HERPES SIMPLEX VIRUS
9.5.1 DNA POLYMERASE UL30
9.5.1.1 ACICLOVIR
9.5.1.2 FAMCICLOVIR
9.5.1.3 VALACICLOVIR
9.5.1.4 PENCICLOVIR TRIFLURIDINE
9.5.1.5 BRIVUDINE
9.5.1.6 FOSCARNET
9.5.1.7 IDOXURIDINE
9.5.2 ENVELOPE PROTEINS
9.5.2.1 DOCOSANOL
9.5.2.2 OTHERS
9.6 HUMAN CYTOMEGALOVIRUS (HCMV)
9.6.1 GANCICLOVIR
9.6.2 VALGANCICLOVIR
9.6.3 CIDOFOVIR
9.6.4 FOSCARNET
9.6.5 FOMIVIRSEN
9.7 VARICELLA-ZOSTER VIRUS (VZV)
9.7.1 VALACICLOVIR
9.7.2 FAMCICLOVIR
9.7.3 ACICLOVIR
9.7.4 VIDARABINE
9.7.5 BRIVUDINE
9.8 HEPATITIS B VIRUS
9.8.1 ENTECAVIR
9.8.2 TENOFOVIR
9.8.3 TELBIVUDINE
9.8.4 TENOFOVIR ALAFENAMIDE
9.8.5 OTHERS
9.9 RESPIRATORY SYNCYTIAL VIRUS
9.9.1 RNA POLYMERASE
9.9.1.1 RIBAVIRIN
9.9.1.2 OTHERS
9.9.2 FUSION GLYCOPROTEIN
9.9.2.1 PALIVIZUMAB
9.9.2.2 OTHERS
9.1 CORONAVIRUS INFECTION
9.11 OTHERS
10 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 GERIATRIC
10.2.1 MALE
10.2.2 FEMALE
10.3 CHILD
10.3.1 MALE
10.3.2 FEMALE
10.4 ADULT
10.4.1 MALE
10.4.2 FEMALE
11 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS
11.1 OVERVIEW
11.2 ORAL
11.2.1 SOLID
11.2.1.1 TABLETS
11.2.1.2 CAPSULES
11.2.1.3 OTHERS
11.2.2 SEMISOLID
11.2.2.1 GELS
11.2.2.2 EMULSIONS
11.2.2.3 ELIXIRS
11.2.2.4 OTHERS
11.2.3 LIQUID
11.2.3.1 SOLUTIONS
11.2.3.2 SYRUPS
11.2.3.3 OTHERS
11.3 TOPICAL
11.3.1 SEMI-SOLID
11.3.1.1 CREAM
11.3.1.2 OINTMENT
11.3.1.3 GELS
11.3.1.4 OTHERS
11.3.2 LIQUID
11.3.2.1 SOLUTIONS
11.3.2.2 SUSPENSIONS
11.3.3 SOLID
11.3.3.1 POWDERS
11.3.3.2 SUPPOSITORIES
11.3.3.3 ENEMA
11.3.3.4 OTHERS
11.4 PARENTERAL
11.4.1 CONVENTIONAL DRUG DELIVERY FORMUALTIONS
11.4.1.1 SOLUTIONS
11.4.1.2 RECONSTITUTED/LYOPHILIZED
11.4.1.3 SUSPENSIONS
11.4.1.4 EMULSIONS
11.4.1.5 OTHERS
11.4.2 NOVEL DRUG DELIVERY FORMULATIONS
11.4.3 COLLOIDAL DISPERSIONS
11.4.4 LONG ACTING INJECTION FORMULATION
12 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERIC
12.3 BRANDED
13 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CENTERS
13.4 AMBULATORY CENTRES
13.5 CLINICS
13.6 HOME HEALTHCARE
13.7 OTHERS
14 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 GILEAD SCIENCES, INC. (2022)
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 PFIZER INC. (2022)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 SIGA TECHNOLOGIES (2022)
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 GLAXOSMITHKLINE PLC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENT
18.5 F. HOFFMANN-LA ROCHE LTD. (2022)
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBVIE INC.
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 AUROBINDO PHARMA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 AVET PHARMACEUTICALS INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BRISTOLL MYERS SQUIBB (2022)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BIOCRYST PHARMACEUTICALS, INC. (2022)
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 CIPLA INC. (2022)
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENT
18.12 DR. REDDY’S LABORATORIES LTD. (2022)
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 EMERGENT (2022)
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 HETERO.
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENT
18.15 JOHNSON & JOHNSON PRIVATE LIMITED (2022)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MACLEODS PHARMACEUTICALS LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MERCK & CO., INC, (2022)
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENT
18.18 MYLAN N.V (SUBSIDIARY OF VIATRIS) (2022)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 NAVINTA LLC.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 SUN PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 TEVA PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENT
18.22 ZYDUS PHARMACEUTICALS, INC. (2022)
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021- 2030 (USD MILLION)
TABLE 2 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA HUMAN CYTOMEGALOVIRUS (HCMV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA HEPATITIS B VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA RESPIRATORY SYNCYTIAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA CORONAVIRUS INFECTION IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021- 2030 (USD MILLION)
TABLE 42 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCT, 2021- 2030 (USD MILLION)
TABLE 49 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021- 2030 (USD MILLION)
TABLE 64 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021- 2030 (USD MILLION)
TABLE 65 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021- 2030 (USD MILLION)
TABLE 66 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 83 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 84 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 86 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 87 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 98 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 99 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 100 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 101 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 102 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 103 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 104 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 105 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 106 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 107 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 108 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 109 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 110 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 111 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 112 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 113 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 114 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 115 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 116 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 117 U.S. ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 118 U.S. INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.S. NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 120 U.S. M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 121 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 122 U.S. HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 123 U.S. REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 124 U.S. NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 125 U.S. NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 126 U.S. INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 127 U.S. NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 128 U.S. INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 129 U.S. GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 130 U.S. PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 131 U.S. HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 132 U.S. NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 133 U.S. NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 134 U.S. NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 135 U.S. NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 136 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 137 U.S. MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 138 U.S. HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 139 U.S. DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 140 U.S. ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 141 U.S. HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 142 U.S. VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 143 U.S. DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 144 U.S. RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 145 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 146 U.S. FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 147 U.S. ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 148 U.S. GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 149 U.S. CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 150 U.S. ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 151 U.S. ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 152 U.S. ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 153 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 154 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 155 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 156 U.S. TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 157 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 158 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 159 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 160 U.S. PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 161 U.S.CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 162 U.S. NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 163 U.S. ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 164 U.S. ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 U.S. ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 CANADA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 167 CANADA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 168 CANADA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 169 CANADA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 170 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 171 CANADA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 172 CANADA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 173 CANADA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 174 CANADA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 175 CANADA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 176 CANADA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 177 CANADA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 178 CANADA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 179 CANADA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 180 CANADA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 181 CANADA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 182 CANADA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 183 CANADA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 184 CANADA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 185 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 186 CANADA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 187 CANADA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 188 CANADA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 189 CANADA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 190 CANADA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 191 CANADA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 192 CANADA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 193 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 194 CANADA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 195 CANADA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 196 CANADA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 197 CANADA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 198 CANADA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 199 CANADA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 200 CANADA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 201 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 202 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 203 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 204 CANADA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 205 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 206 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 207 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 208 CANADA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 209 CANADA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 210 CANADA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 211 CANADA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 212 CANADA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 CANADA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 MEXICO ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 215 MEXICO INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 216 MEXICO NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 217 MEXICO M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 218 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 219 MEXICO HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 220 MEXICO REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 221 MEXICO NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 222 MEXICO NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 223 MEXICO INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 224 MEXICO NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 225 MEXICO INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 226 MEXICO GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 227 MEXICO PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 228 MEXICO HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 229 MEXICO NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 230 MEXICO NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 231 MEXICO NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 232 MEXICO NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 233 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 234 MEXICO MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 235 MEXICO HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 236 MEXICO DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 237 MEXICO ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 238 MEXICO HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 239 MEXICO VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 240 MEXICO DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 241 MEXICO RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 242 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 243 MEXICO FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 244 MEXICO ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 245 MEXICO GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 246 MEXICO CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 247 MEXICO ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 248 MEXICO ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 249 MEXICO ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 250 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 251 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 252 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 253 MEXICO TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 254 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 255 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 256 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 257 MEXICO PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 258 MEXICO CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 259 MEXICO NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 260 MEXICO ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 261 MEXICO ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 262 MEXICO ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA ANTIVIRAL DRUGS: SEGMENTATION
FIGURE 2 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA ANTIVIRAL DRUGS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ANTIVIRAL DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ANTIVIRAL DRUGS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA ANTIVIRAL DRUGS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE OF VIRAL INFECTIONS IS EXPECTED TO DRIVE THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INFLUENZA SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
FIGURE 14 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2022
FIGURE 15 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , CAGR (2023-2030)
FIGURE 17 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , LIFELINE CURVE
FIGURE 18 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2022
FIGURE 19 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2022
FIGURE 23 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2022
FIGURE 27 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2022
FIGURE 31 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 35 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SNAPSHOT (2022)
FIGURE 39 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022)
FIGURE 40 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION (2023-2030)
FIGURE 43 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.