North America Cancer Spit Test Device Market
Market Size in USD Million
CAGR :
% 
 USD
370.51 Million
USD
794.22 Million
2025
2033
USD
370.51 Million
USD
794.22 Million
2025
2033
| 2026 –2033 | |
| USD 370.51 Million | |
| USD 794.22 Million | |
|
|
|
|
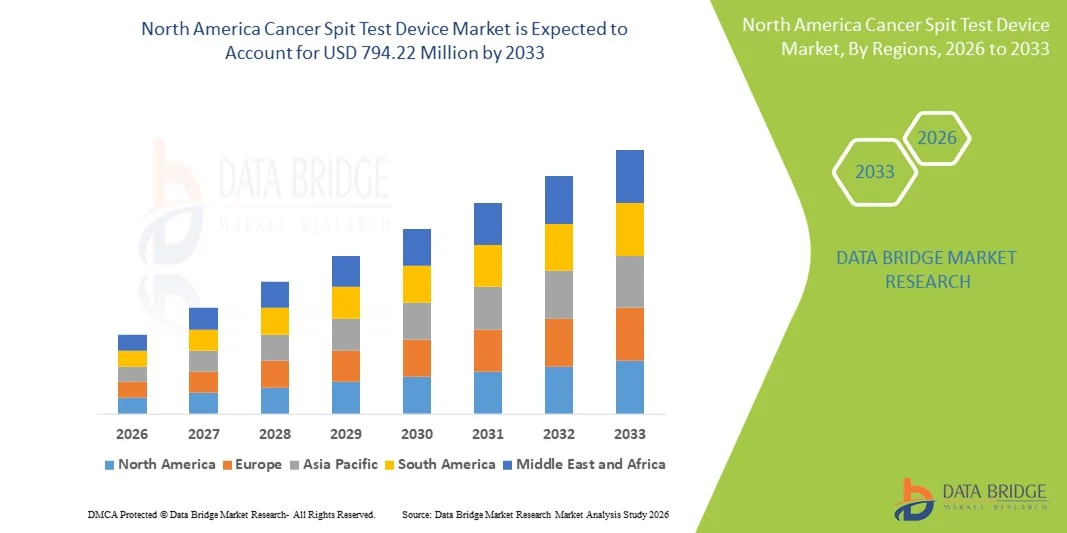
North America Cancer Spit Test Device Market Size
- The North America Cancer Spit Test Device market size was valued at USD 370.51 Million in 2025 and is expected to reach USD 794.22 Million by 2033, at a CAGR of 10.00% during the forecast period
- The market growth is largely fueled by the rising adoption of non-invasive diagnostic technologies and continuous technological advancements in saliva-based testing devices, leading to increased acceptance of cancer spit test devices across clinical and research settings
- Furthermore, growing demand for early cancer detection, patient-friendly collection methods, and cost-effective diagnostic solutions is establishing cancer spit test devices as a preferred alternative to traditional invasive testing methods. These converging factors are accelerating the uptake of cancer spit test device solutions, thereby significantly boosting overall market growth
North America Cancer Spit Test Device Market Analysis
- Cancer spit test devices, which enable non-invasive, saliva-based sample collection for cancer screening and monitoring, are becoming increasingly important diagnostic tools across hospitals, diagnostic laboratories, and oncology research settings due to their patient-friendly nature, ease of collection, and compatibility with advanced molecular and biomarker-based testing technologies
- The rising demand for cancer spit test devices is primarily driven by the growing emphasis on early cancer detection, increasing prevalence of various cancers, and a strong preference for non-invasive, cost-effective, and easy-to-administer diagnostic solutions among both patients and healthcare providers
- U.S. dominated the cancer spit test device market with the largest revenue share of approximately 39.2% in 2025, supported by its advanced healthcare infrastructure, high adoption of innovative diagnostic technologies, strong government and private investments in cancer screening programs, and presence of leading market players and research institutions
- Canada is expected to be the fastest-growing country in the cancer spit test device market during the forecast period, registering a CAGR of 20.8%, driven by rising cancer awareness, expanding diagnostic and research facilities, increasing healthcare investments, and growing focus on early detection through non-invasive testing methods
- The adult segment dominated the largest market revenue share of 78.4% in 2025, driven by higher cancer prevalence among adults and elderly populations
Report Scope and Cancer Spit Test Device Market Segmentation
|
Attributes |
Cancer Spit Test Device Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
North America Cancer Spit Test Device Market Trends
Rising Adoption of Non-Invasive and Early Cancer Screening Solutions
- A significant and accelerating trend in the cancer spit test device market is the growing preference for non-invasive, saliva-based diagnostic technologies for early cancer detection. These devices offer a painless and convenient alternative to blood tests and tissue biopsies, improving patient comfort and screening compliance across various age groups
- For instance, several diagnostic companies are developing saliva-based testing platforms capable of detecting biomarkers associated with oral, lung, breast, and gastrointestinal cancers, enabling earlier identification of disease risk and improving preventive healthcare outcomes
- Cancer spit test devices support rapid sample collection and simplified testing procedures, making them suitable for routine screenings, outpatient settings, and large-scale population health programs. This ease of use is particularly beneficial for individuals who are reluctant to undergo invasive diagnostic procedures
- The increasing focus on preventive healthcare and regular health check-ups is further supporting the adoption of saliva-based cancer testing devices, especially in regions with rising cancer prevalence and growing healthcare awareness
- This trend toward accessible, patient-friendly diagnostic solutions is reshaping expectations for cancer screening, encouraging healthcare providers to incorporate saliva-based testing into early detection strategies and clinical workflows
- As a result, the demand for cancer spit test devices is steadily increasing across hospitals, diagnostic laboratories, and home-based testing environments, supporting overall market growth
North America Cancer Spit Test Device Market Dynamics
Driver
Growing Emphasis on Early Cancer Detection and Preventive Healthcare
- The increasing global incidence of cancer and the growing awareness of the benefits of early-stage diagnosis are major drivers fueling the demand for cancer spit test devices. Early detection significantly improves treatment success rates and reduces long-term healthcare costs
- For instance, public health initiatives and cancer screening programs in several countries are encouraging the adoption of non-invasive diagnostic tools to facilitate early identification of high-risk individuals and improve survival outcomes
- Cancer spit test devices enable frequent and repeatable testing, supporting ongoing monitoring and early intervention without causing discomfort to patients. This makes them particularly suitable for preventive screening and follow-up assessments
- In addition, rising healthcare expenditure, expanding oncology research, and increasing investment in diagnostic innovation are further accelerating market adoption across both developed and emerging economies
- The convenience of easy sample collection, faster test results, and reduced dependency on specialized infrastructure continues to propel the uptake of cancer spit test devices in clinical and non-clinical settings
Restraint/Challenge
Concerns Related to Diagnostic Accuracy, Regulatory Approval, and Cost
- Concerns regarding the diagnostic accuracy and clinical reliability of cancer spit test devices pose a significant challenge to broader market adoption. As cancer diagnosis requires high precision, false-positive or false-negative results can limit confidence among healthcare professionals and patients
- For instance, regulatory authorities require extensive clinical validation and performance data before approving cancer diagnostic devices, which can prolong product development timelines and delay market entry
- In addition, the relatively high cost associated with advanced biomarker research, testing kits, and device development can restrict adoption, particularly in cost-sensitive healthcare systems and developing regions
- While technological advancements are gradually improving affordability, the perceived cost compared to conventional diagnostic methods can still hinder widespread use, especially where reimbursement frameworks are limited or unclear
- Overcoming these challenges through improved clinical validation, regulatory clarity, cost optimization, and increased awareness among healthcare providers will be essential for sustaining long-term growth in the cancer spit test device market
North America Cancer Spit Test Device Market Scope
The market is segmented on the basis of distribution channel, product type, site of collection, end user, method of collection, age group, and application.
- By Distribution Channel
On the basis of distribution channel, the Cancer Spit Test Device market is segmented into retail sales, direct tender, and others. The direct tender segment dominated the largest market revenue share of 44.6% in 2025, driven by large-scale procurement by government hospitals and public healthcare systems. Direct tenders allow bulk purchase of cancer spit test devices at reduced costs, making them ideal for national screening programs. These tenders also ensure standardized product quality across institutions. Hospitals and diagnostic centers prefer tender-based procurement due to regulatory compliance and long-term supply contracts. Public health initiatives focused on early cancer detection further strengthen demand for this channel. Many developing countries are increasing budgets for cancer screening, which increases tender purchases. Manufacturers also favor this channel due to stable revenue and predictable order volumes. The tender process supports large-volume manufacturing and lowers per-unit costs. Government contracts also encourage innovation and product validation. Standardized procurement through tenders ensures device availability in remote regions. This makes direct tender the dominant distribution channel for cancer spit test devices.
The retail sales segment is anticipated to witness the fastest growth rate of 21.3% CAGR from 2026 to 2033, driven by increasing consumer awareness about non-invasive cancer diagnostics. Consumers are increasingly preferring saliva-based tests due to ease and privacy. Retail sales through pharmacies and online stores provide faster access to testing kits. Growth in e-commerce and home diagnostics platforms further supports this segment. Rising preventive healthcare spending is also boosting retail adoption. Many patients prefer self-collection at home rather than hospital visits. Retail sales also benefit from improved packaging and user-friendly instructions. Direct-to-consumer diagnostic models are gaining traction globally. Increasing availability of saliva collection kits in retail stores increases market penetration. Retail channels also support faster product launches and wider reach. The retail segment is expanding rapidly in urban and semi-urban areas. The convenience and privacy offered by retail sales will continue to drive growth.
- By Product Type
On the basis of product type, the Cancer Spit Test Device market is segmented into saliva cryostorage box, bar-code labels, oral swab, fluid-specific devices, saliva collection kit, and others. The saliva collection kit segment dominated the largest market revenue share of 38.9% in 2025, driven by its integrated design combining collection, preservation, and transport. These kits maintain sample stability and reduce contamination risks. They are widely used in hospitals and diagnostic labs due to high reliability. The kits also provide standardized procedures, improving test accuracy. Saliva collection kits are compatible with molecular diagnostics and biomarker analysis, making them preferred in oncology. High adoption in cancer screening programs supports market dominance. Clinical trials and research studies often require standardized collection kits. Manufacturers continuously improve kit designs to increase user convenience. These kits also support remote sample collection and mail-in testing. Rising demand for non-invasive testing increases kit adoption. High acceptance among patients further strengthens this segment. The saliva collection kit remains the dominant product type due to its convenience and accuracy.
The oral swab segment is expected to witness the fastest CAGR of 22.1% from 2026 to 2033, supported by its non-invasive nature and patient comfort. Oral swabs allow quick and painless sample collection. They are highly suitable for large-scale screening and mass testing programs. Adoption in pediatric and geriatric populations is increasing due to ease of use. Oral swabs also require minimal training for collection, reducing operational costs. They are suitable for outpatient and home-based testing. Technological advancements improving biomarker recovery from swabs increase reliability. Lower cost compared to full collection kits supports growth. Increasing use in remote regions where lab access is limited also drives demand. Oral swabs are becoming popular for quick screening before advanced testing. Rising awareness of early cancer detection boosts adoption. Overall, the oral swab segment is expected to grow rapidly due to convenience and low cost.
- By Site of Collection
On the basis of site of collection, the Cancer Spit Test Device market is segmented into minor salivary gland, parotid gland, and sub-mandibular/sub-lingual gland. The parotid gland segment dominated the largest market revenue share of 41.7% in 2025, driven by high-quality saliva production suitable for biomarker analysis. Saliva from the parotid gland is considered highly reliable for molecular diagnostics. Strong adoption in hospitals and research institutions supports dominance. The parotid gland also provides higher saliva volume compared to other sites. Standardized collection methods improve sample accuracy. Higher output makes testing more efficient and scalable. This site is preferred in clinical trials due to consistency. Increased clinical validation further strengthens its position. The parotid gland is widely used in advanced oncology research. Laboratories prefer this site for its reliability and repeatability. The site’s dominance is reinforced by long-term use in diagnostic workflows. This makes parotid gland the leading collection site for cancer spit tests.
The minor salivary gland segment is anticipated to witness the fastest growth rate of 20.8% CAGR from 2026 to 2033, driven by advancements in micro-sampling and minimally invasive collection. This site is gaining attention for localized cancer detection, especially in oral cancers. The minor glands offer specific biomarker profiles beneficial for early detection. Increased research focus on localized cancer diagnostics supports adoption. Clinical studies are validating the effectiveness of minor gland sampling. Improved collection devices make sampling easier and more reliable. This site is also preferred for targeted testing in high-risk populations. Rising demand for precision diagnostics further accelerates growth. Expansion of personalized oncology treatment increases use of minor gland samples. The segment is also growing due to its non-invasive nature. Adoption is rising in both hospitals and research labs. Overall, the minor salivary gland segment is expected to grow rapidly due to technological advancements and clinical validation.
- By End User
On the basis of end user, the Cancer Spit Test Device market is segmented into hospitals, diagnostic laboratories, oncology specialty clinics, cancer research institutes, and others. The diagnostic laboratories segment accounted for the largest market revenue share of 36.5% in 2025, driven by high testing volumes and advanced molecular diagnostic infrastructure. Diagnostic labs serve as central hubs for cancer screening and confirmation. They offer high-throughput testing capabilities required for large-scale programs. Strong investments in automation and AI-based diagnostics support dominance. Collaboration with hospitals and research institutes increases test volume. Diagnostic labs also provide specialized biomarker analysis, enhancing accuracy. Growing demand for early cancer detection further supports this segment. Many labs are expanding their molecular diagnostic services. Increased outsourcing of testing from hospitals boosts lab revenues. Rising use of saliva-based diagnostics in labs increases adoption. Diagnostic labs also support clinical trials and research studies. Overall, diagnostic laboratories remain the dominant end-user segment.
The oncology specialty clinics segment is expected to witness the fastest CAGR of 23.4% from 2026 to 2033, driven by rising number of specialized cancer care centers globally. These clinics increasingly adopt saliva-based tests for patient comfort and rapid screening. Patients prefer non-invasive tests in outpatient settings. Clinics also benefit from quick diagnostic results and improved patient compliance. Personalized cancer care and targeted treatment increase demand for rapid diagnostics. Specialty clinics are expanding in developing markets due to rising cancer cases. Growing awareness and screening programs also support growth. Clinics often partner with diagnostic labs for confirmatory testing. Increasing adoption of advanced diagnostic tools in clinics accelerates growth. Continuous improvement in spit test devices supports clinical adoption. Overall, oncology specialty clinics are expected to grow rapidly due to patient demand and technological advancement.
- By Method of Collection
On the basis of method of collection, the Cancer Spit Test Device market is segmented into oral swab, passive drool, and others. The passive drool segment dominated the largest market revenue share of 39.2% in 2025, driven by its ability to collect larger saliva volumes with minimal contamination. Passive drool provides high-quality samples suitable for genomic and proteomic analysis. This method is widely used in laboratory and research environments. It supports standardized testing procedures, improving accuracy. Passive drool is also cost-effective compared to complex collection methods. Many clinical trials prefer this method due to sample reliability. The method is easy for patients and requires minimal training. It also supports large-scale screening due to simplicity. Growing use in hospitals and diagnostic centers reinforces dominance. Strong compatibility with advanced biomarker analysis supports continued adoption. Overall, passive drool remains the leading collection method.
The oral swab segment is anticipated to witness the fastest growth rate of 22.7% CAGR from 2026 to 2033, driven by its simplicity and patient-friendly nature. Oral swabs allow quick collection without specialized training. They are ideal for home-based testing and remote areas. Increased use in pediatric and geriatric populations supports growth. Technological improvements in swab materials increase biomarker capture efficiency. Oral swabs are also cost-effective and easy to transport. Rising demand for rapid screening supports adoption. Many healthcare providers prefer swabs for outpatient testing. Growing awareness of early cancer detection increases usage. The oral swab segment benefits from expanding e-commerce and retail availability. Overall, oral swabs are expected to grow rapidly due to convenience and low cost.
- By Age Group
On the basis of age group, the Cancer Spit Test Device market is segmented into pediatrics and adult. The adult segment dominated the largest market revenue share of 78.4% in 2025, driven by higher cancer prevalence among adults and elderly populations. Most cancer screening programs target adult demographics. Routine health checkups and preventive screenings are more common among adults. Increased awareness of early detection drives higher testing volumes. Adults also have higher access to diagnostic services. Higher healthcare spending among adults supports market dominance. Many cancer screening policies focus on adult age groups. Saliva-based diagnostics are widely accepted in adult oncology. Increased adoption of molecular testing further strengthens the segment. Aging population globally continues to increase demand. Overall, the adult segment remains dominant due to higher disease prevalence and screening focus.
The pediatric segment is expected to witness the fastest CAGR of 21.9% from 2026 to 2033, driven by increasing emphasis on non-invasive testing for children. Saliva-based tests are preferred due to minimal discomfort and easier sample collection. Pediatric oncology research is growing, supporting demand for new diagnostic tools. Parents increasingly seek painless testing options for children. Pediatric hospitals and clinics are adopting saliva-based diagnostics. Rising awareness of early cancer detection in children also drives growth. Technological advancements make saliva tests more reliable for pediatric use. Increased government and private funding supports pediatric diagnostic development. Expansion of pediatric specialty centers boosts adoption. The pediatric segment is expected to grow rapidly due to rising demand and technological improvements.
- By Application
On the basis of application, the Cancer Spit Test Device market is segmented into breast cancer, colon and rectal cancer, prostate cancer, pancreatic cancer, oral cancer, thyroid cancer, endometrial cancer, kidney cancer, leukemia, melanoma, non-Hodgkin lymphoma, liver-lung cancer, and others. The breast cancer segment dominated the largest market revenue share of 19.6% in 2025, driven by high global incidence and extensive screening programs. Saliva-based biomarkers for breast cancer are well researched and validated. Non-invasive testing improves patient participation and compliance. Government initiatives and awareness campaigns increase screening rates. Breast cancer diagnostics benefit from high funding and research focus. Increasing adoption of personalized medicine supports demand. Saliva-based tests provide early detection options and reduce clinical burden. Many healthcare institutions integrate saliva tests into screening workflows. Continuous clinical trials validate effectiveness and improve adoption. Rising patient awareness further boosts demand. Breast cancer remains the leading application due to prevalence and research focus.
The pancreatic cancer segment is anticipated to witness the fastest growth rate of 24.1% CAGR from 2026 to 2033, driven by the urgent need for early detection due to high mortality rates. Pancreatic cancer currently has limited early screening options. Saliva-based biomarkers show promise for early diagnosis. Increasing research investments and clinical trials support development. Rising demand for innovative diagnostic tools accelerates adoption. Improved sensitivity and specificity of saliva tests increase clinical acceptance. Growing collaborations between research institutes and diagnostic companies further support growth. Expansion of precision oncology programs also contributes. Increased awareness of pancreatic cancer risks drives screening. Early detection can significantly improve survival rates. As a result, pancreatic cancer is expected to be the fastest-growing application segment.
North America Cancer Spit Test Device Market Regional Analysis
- The North America cancer spit test device market is expected to witness robust growth during the forecast period of 2026 to 2033, driven by rising cancer prevalence, increasing awareness of early disease detection, and expanding access to healthcare services across the region
- Rapid improvements in diagnostic technologies, coupled with growing government focus on preventive healthcare and cancer screening programs, are accelerating the adoption of saliva-based cancer testing solutions across North America
- The North America region is emerging as a key hub for diagnostic research, manufacturing, and clinical validation, which is enhancing the affordability and availability of cancer spit test devices. The growing emphasis on early diagnosis, reduced patient discomfort, and cost-effective screening solutions is supporting widespread adoption across hospitals, diagnostic laboratories, and community healthcare centers
U.S. Cancer Spit Test Device Market Insight
The U.S. cancer spit test device market dominated North America with the largest revenue share of approximately 39.2% in 2025, supported by its advanced healthcare infrastructure, high adoption of innovative diagnostic technologies, and strong government and private investments in cancer screening programs. The presence of leading market players, extensive clinical research institutions, and strong funding for oncology research is driving the adoption of saliva-based cancer testing solutions in the U.S. In addition, the increasing focus on early cancer detection, preventive healthcare, and expanded reimbursement policies are contributing to the growing demand for non-invasive cancer screening methods across hospitals, diagnostic labs, and outpatient facilities.
Canada Cancer Spit Test Device Market Insight
Canada cancer spit test device market is expected to be the fastest-growing country in the North America cancer spit test device market during the forecast period, registering a CAGR of 20.8%, driven by rising cancer awareness, expanding diagnostic and research facilities, increasing healthcare investments, and growing focus on early detection through non-invasive testing methods. The country’s healthcare system and supportive public health initiatives are encouraging the adoption of saliva-based cancer diagnostic solutions across clinical and community healthcare settings. Moreover, the growing emphasis on preventive healthcare, increasing government funding for cancer research, and the expansion of diagnostic laboratories are accelerating market growth in Canada, positioning it as a key growth market in North America.
North America Cancer Spit Test Device Market Share
The Cancer Spit Test Device industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific (U.S.)
- QIAGEN (Germany)
- Roche Diagnostics (Switzerland)
- Danaher Corporation (U.S.)
- Agilent Technologies (U.S.)
- Illumina (U.S.)
- Bio-Rad Laboratories (U.S.)
- Merck KGaA (Germany)
- Becton, Dickinson and Company (BD) (U.S.)
- OraSure Technologies (U.S.)
- Salimetrics (U.S.)
- DNA Genotek (Canada)
- Takara Bio (Japan)
- Norgen Biotek (Canada)
- Genotek Bioproducts (U.S.)
- Abcam (U.K.)
- Zymo Research (U.S.)
- Promega Corporation (U.S.)
- NeoGenomics Laboratories (U.S.)
- BGI Genomics (China)
Latest Developments in North America Cancer Spit Test Device Market
- In January 2021, DNA Genotek announced that the U.S. Food and Drug Administration (FDA) granted general use 510(k) clearance for its OrageneDx saliva collection products, enhancing regulatory confidence and enabling broader clinical use of saliva-based DNA testing technologies as part of early disease detection workflows
- In October 2023, researchers at the University of California, Los Angeles (UCLA) developed a saliva-based test capable of detecting early-stage oral and throat cancers with over 90% accuracy, marking a significant advance in non-invasive cancer screening and expanding the potential application of spit diagnostics for head and neck cancers
- In October 2024, a new at-home saliva test for prostate cancer was introduced by a research team led by The Institute of Cancer Research in London, designed to identify genetic risk scores for prostate cancer and potentially save the NHS £500 million annually by enabling earlier diagnosis of aggressive disease, demonstrating strong health-economic value for spit-based cancer diagnostics
- In February 2024, scientists from the University of Florida and National Yang Ming Chiao Tung University in Taiwan reported a hand-held biosensor that can detect breast cancer biomarkers from saliva in under five seconds, offering a rapid, portable approach to early breast cancer screening
- In May 2024, Israeli biotech firm Salignostics partnered with Sheba Medical Center’s ARC Innovation to develop an early oral cavity cancer diagnostic test using saliva samples, aiming to create one of the first commercial saliva-based tests for oral cancer detection
- In April 2025, researchers published results showing that an at-home spit test using a polygenic risk score could outperform traditional PSA blood tests in identifying prostate cancer risk, highlighting the growing clinical validation of saliva-based genetic screening tools for cancer risk assessment
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.