North America Capnography Consumables Market
Market Size in USD Million
CAGR :
% 
 USD
162.68 Million
USD
249.66 Million
2024
2032
USD
162.68 Million
USD
249.66 Million
2024
2032
| 2025 –2032 | |
| USD 162.68 Million | |
| USD 249.66 Million | |
|
|
|
|
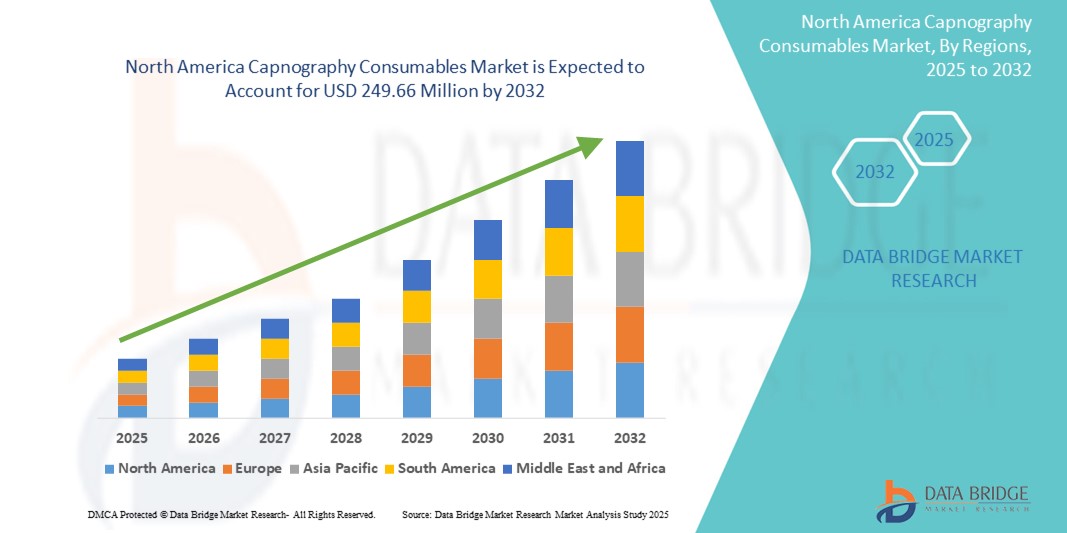
North America Capnography Consumables Market Size
- The North America capnography consumables market size was valued at USD 162.68 million in 2024 and is expected to reach USD 249.66 million by 2032, at a CAGR of 5.50% during the forecast period
- The market growth is largely fueled by the increasing incidence of respiratory disorders such as chronic obstructive pulmonary disease (COPD), asthma, and obstructive sleep apnea across North America, driving the demand for effective patient monitoring solutions like capnography
- Furthermore, the rising number of surgical procedures and emergency medical interventions, where capnography is considered a standard of care for airway and ventilation monitoring, is significantly contributing to the uptake of capnography consumables across hospitals and ambulatory surgical centers
North America Capnography Consumables Market Analysis
- Capnography consumables, including sampling lines, adapters, and filters, are increasingly vital components of respiratory monitoring systems in both hospital and pre-hospital settings due to their critical role in measuring end-tidal carbon dioxide (EtCO₂) for effective ventilation assessment and patient safety
- The escalating demand for capnography consumables is primarily fueled by the rising prevalence of respiratory disorders, increasing surgical procedures requiring anesthesia, and the growing adoption of capnography monitoring in emergency care and non-invasive settings across North America
- U.S. dominated the North America capnography consumables market with the largest market share of 78.4% in 2024, owing to well-established hospital networks, widespread use of capnography in procedural sedation and pain management, and rapid integration of advanced consumables in clinical settings. Furthermore, strong regulatory support and the presence of leading market players drive innovation and accessibility
- Mexico is projected to be the fastest-growing market in North America for capnography consumables during the forecast period (2025–2032), driven by expanding healthcare access, government initiatives to improve surgical and emergency care, and increased awareness of the benefits of respiratory monitoring. The country's efforts to strengthen its public hospital infrastructure and train paramedical personnel are expected to significantly enhance the use of capnography devices and associated consumables
- The OEM modules segment dominated the North America capnography consumables market with a revenue share of 52.4% in 2024, primarily due to the rising demand from medical device manufacturers who integrate capnography technology into broader patient monitoring systems
Report Scope and North America Capnography Consumables Market Segmentation
|
Attributes |
North America Capnography Consumables Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Capnography Consumables Market Trends
“Advancing Clinical Efficiency Through AI-Driven Capnography Solutions”
- A significant and accelerating trend in the North America capnography consumables market is the deepening integration with artificial intelligence (AI) and advanced monitoring systems, significantly enhancing clinical accuracy and workflow efficiency in healthcare settings
- For instance, modern capnography consumables such as AI-integrated sensor and sensor cable systems are enabling real-time analytics in intensive care units (ICUs) and operating rooms. These systems are capable of automatically adjusting alarms or recommending interventions based on patient-specific CO₂ trends, thereby improving care outcomes
- AI-based modules can also detect abnormal breathing patterns, facilitating early intervention in trauma and emergency care. Additionally, some OEM modules now feature intelligent connectivity with Electronic Health Records (EHR), ensuring seamless documentation and reducing manual entry errors
- The seamless integration of capnography consumables with digital hospital systems facilitates better collaboration among clinical teams. With unified interfaces, professionals can manage capnography data alongside ECG, oxygen saturation, and other critical parameters to streamline patient care
- This trend toward more intelligent, responsive, and interconnected monitoring systems is redefining respiratory diagnostics. Companies such as Medtronic and Masimo are at the forefront, developing capnography consumables that support AI-powered analytics and cloud-based telemetry to deliver real-time insights
- The demand for capnography consumables with smart integration is growing rapidly across trauma centers, ambulatory care units, and homecare settings as healthcare providers prioritize both accuracy and operational efficiency in critical patient monitoring
North America Capnography Consumables Market Dynamics
Driver
“Growing Need Due to Increasing Demand for Accurate and Continuous Respiratory Monitoring”
- The growing prevalence of respiratory conditions such as COPD, asthma, and acute respiratory distress, particularly among aging populations, is significantly driving the demand for capnography consumables across North America. Hospitals and emergency care providers are increasingly relying on capnography masks, cannulas, and sensor kits to provide real-time patient monitoring, particularly in intensive care and procedural sedation
- For instance, in April 2024, Masimo Corporation introduced a new range of sidestream capnography consumables designed for pediatric patients, aiming to improve comfort and accuracy in non-invasive CO₂ monitoring. Innovations like this are expected to further fuel market growth by expanding the range of clinical applications
- The increasing integration of capnography modules in advanced patient monitoring systems across emergency rooms, operating rooms, and home care settings has significantly improved diagnostic precision. Real-time monitoring of ventilation status through capnography consumables enables quicker interventions, reducing complications and improving patient outcomes
- In addition, the rise in same-day outpatient surgeries and home-based critical care is increasing demand for portable and disposable capnography accessories, such as cannulas and sensor cables, due to their convenience and hygiene benefits. The ongoing digitalization of healthcare and the demand for early warning systems in patient deterioration are also strengthening the role of capnography in modern medicine
Restraint/Challenge
“High Cost of Advanced Consumables and Integration Complexities”
- The high cost of disposable capnography consumables, especially for advanced multi-channel or pediatric-specific products, remains a key challenge for smaller healthcare facilities and budget-constrained providers. This restricts broader adoption across rural and low-volume centers in North America
- For instance, bulk procurement of disposable capnography sensors and adapter kits for high-volume hospitals incurs significant costs, particularly for continuous monitoring in intensive care or trauma care units. As a result, cost-efficiency becomes a critical purchasing factor
- Moreover, integration challenges between legacy monitoring systems and new capnography modules or OEM components can result in compatibility issues and additional installation costs. This creates hesitation among providers looking to scale their monitoring infrastructure
- To overcome these challenges, leading manufacturers such as Medtronic, Masimo, and Philips are focusing on offering cost-effective consumables, bundling deals for multi-use equipment, and designing universal adapters that ensure system compatibility. In addition, government reimbursement support and value-based healthcare models in the U.S. are helping improve affordability and adoption across public and private settings
- Continued investments in R&D for reusable or semi-reusable consumables, and strategic partnerships between manufacturers and healthcare providers, will be key to reducing per-use costs while maintaining clinical efficacy in the North America capnography consumables market
North America Capnography Consumables Market Scope
The market is segmented on the basis of product, component, application, end user, and distribution channel.
- By Product
On the basis of product, the North America capnography consumables market is segmented into capnography mask, sensor and sensor cable, adapter kit, cannula, and others. The cannula segment dominated the largest market revenue share of 37.5% in 2024, owing to its widespread usage in delivering capnography monitoring across various clinical settings. It is a standard accessory in continuous monitoring for patients under anesthesia or sedation.
The sensor and sensor cable segment is projected to witness the fastest CAGR of 19.8% from 2025 to 2032, driven by increasing technological advancements in real-time monitoring and the growing emphasis on accuracy and reliability in clinical diagnostics.
- By Component
On the basis of component, the North America capnography consumables market is segmented into OEM modules and others. The OEM modules segment held the largest market share of 52.4% in 2024, primarily due to the rising demand from medical device manufacturers who integrate capnography technology into broader patient monitoring systems.
The others segment, which includes software and accessories, is expected to grow at the fastest CAGR of 16.1%, due to increasing demand for modular and upgradable monitoring solutions.
- By Application
On the basis of application, the North America capnography consumables market is segmented into cardiac care, trauma and emergency care, respiratory monitoring, sleep monitoring, procedural sedation, and others. The respiratory monitoring segment accounted for the highest revenue share of 29.6% in 2024, supported by the rise in respiratory conditions such as COPD and asthma.
The procedural sedation segment is anticipated to grow at the fastest CAGR of 20.3% due to its expanding role in non-operating room anesthesia and outpatient settings.
- By End User
On the basis of end user, the North America capnography consumables market is segmented into hospitals, specialty clinics, trauma centers, ambulatory care centers, homecare settings, and others. The hospitals segment dominated the market with a share of 48.7% in 2024, attributed to high patient volumes and the availability of advanced monitoring infrastructure.
Meanwhile, the homecare settings segment is poised to register the fastest CAGR of 21.5% from 2025 to 2032, due to the increasing preference for remote monitoring and chronic disease management at home.
- By Distribution Channel
On the basis of distribution channel, the North America capnography consumables market is segmented into direct tender, retail sales, and online sales. The direct tender segment held the largest share of 54.2% in 2024, driven by large-volume purchases by healthcare institutions and government contracts.
The online sales segment is expected to witness the fastest CAGR of 22.8%, fueled by the growing popularity of e-commerce platforms and the need for efficient procurement channels in decentralized care environments.
North America Capnography Consumables Market Regional Analysis
- North America dominated the capnography consumables market with the largest revenue share of 41.2% in 2024, driven by a well-established healthcare infrastructure, strong clinical awareness, and widespread use of capnography in anesthesia and emergency settings. In addition, favorable reimbursement policies and technological integration in patient monitoring devices continue to support growth in this region
- Capnography consumables, including sampling lines, filters, cannulas, and adapters, are essential tools in patient monitoring systems, particularly in surgical settings, emergency care, and intensive care units. These consumables ensure accurate end-tidal CO₂ (EtCO₂) readings, playing a critical role in respiratory assessment and ventilation management
- The growing prevalence of respiratory disorders such as COPD, asthma, and sleep apnea, alongside the rising number of surgical procedures and ICU admissions, is significantly boosting demand for capnography consumables across North America
U.S. Capnography Consumables Market Insight
The U.S. capnography consumables market accounted for the largest share of the North America Capnography Consumables market with a revenue share of 78.4% in 2024, attributed to its advanced healthcare infrastructure, rising number of surgical and trauma cases, and well-established usage guidelines for EtCO₂ monitoring by organizations like the American Society of Anesthesiologists (ASA). The strong presence of key players such as Medtronic, Masimo, and Philips, along with continued investments in critical care and emergency medical services, further support the market’s expansion.
Canada Capnography Consumables Market Insight
The Canada capnography consumables market accounted for the dominant share within North America, holding approximately 58.3% of the regional market in 2024, due to its strong public healthcare system, high adoption of patient monitoring technologies, and stringent clinical guidelines that promote the use of capnography in critical care. The country’s increasing geriatric population and rising incidence of chronic respiratory diseases further amplify the demand for capnography consumables across hospitals and ambulatory settings.
Mexico Capnography Consumables Market Insight
The Mexico capnography consumables market is projected to be the fastest-growing market for capnography consumables in North America during the forecast period (2025–2032), driven by improvements in healthcare infrastructure, government-led modernization of hospitals, and a growing focus on perioperative patient safety. The rising burden of respiratory conditions and the expansion of emergency medical services in both urban and semi-urban areas are propelling the adoption of capnography technology, particularly in public health institutions.
North America Capnography Consumables Market Share
The North America capnography consumables industry is primarily led by well-established companies, including:
- Medtronic (Ireland)
- Koninklijke Philips N.V. (Netherlands)
- Drägerwerk AG & Co. KGaA (Germany)
- Masimo (U.S.)
- BD (U.S.)
- Nihon Kohden Corporation (Japan)
- VYAIRE (U.S.)
- Nonin (U.S.)
- Kingst Commercial and Trade Co., Ltd (China)
- Hamilton Medical (U.S.)
- ZOLL Medical Corporation (U.S.)
- EdanUSA (U.S.)
- Infinium Medical (U.S.)
Latest Developments in North America Capnography Consumables Market
- In April 2024, Masimo Corporation introduced a new line of pediatric sidestream capnography consumables, including masks and sampling lines designed for enhanced comfort and accuracy in children’s respiratory monitoring. This launch is expected to expand clinical applicability in pediatric ICUs and procedural settings
- In July 2024, a study published by Luke’s University Health Network highlighted the impact of Masimo’s SafetyNet remote monitoring solution, which incorporates capnography consumables to monitor discharged patients. This initiative led to significantly reduced hospital readmissions and emergency visits post-joint replacement, showcasing improved patient outcomes
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.