North America Carrier Screening Market
Market Size in USD Billion
CAGR :
% 
 USD
2.32 Billion
USD
4.35 Billion
2024
2032
USD
2.32 Billion
USD
4.35 Billion
2024
2032
| 2025 –2032 | |
| USD 2.32 Billion | |
| USD 4.35 Billion | |
|
|
|
North America Carrier Screening Market Analysis
The North America carrier screening market has experienced significant growth due to advancements in genetic testing technologies and increasing awareness about genetic disorders. The rising prevalence of inherited genetic conditions, along with the growing demand for early detection, is driving market expansion. Healthcare providers and patients are increasingly opting for carrier screening as part of routine healthcare to assess the risk of passing on genetic conditions to offspring.
Key factors influencing the market include improved accuracy and affordability of testing methods, such as next-generation sequencing (NGS), and the availability of comprehensive screening panels covering a wide range of genetic conditions. In addition, growing support from healthcare organizations and government initiatives that promote genetic testing are further accelerating market growth.
The market is also supported by the increasing number of couples and individuals seeking personalized genetic counseling to make informed reproductive decisions. The development of innovative technologies, like expanded carrier screening, is contributing to the rise in adoption rates, as it provides more detailed and actionable insights into a person’s genetic risk profile. Furthermore, the increasing trend towards preventative healthcare and personalized medicine is expected to drive sustained demand for carrier screening services in the coming years.
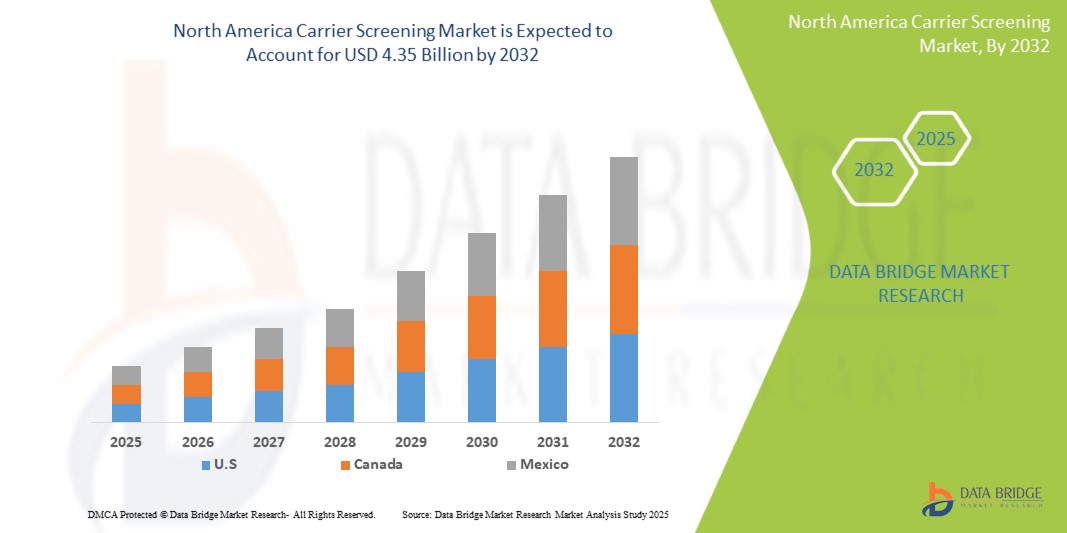
North America Carrier Screening Market Size
The North America carrier screening market size was valued at USD 2.32 billion in 2024 and is projected to reach USD 4.35 billion by 2032, with a CAGR of 7.50% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
North America Carrier Screening Market Trends
“Rise in demand for early detection of genetic disorders”
A key market trend driving the growth of the North American carrier screening market is the rising awareness and demand for early detection of genetic disorders. Advancements in genetic testing technologies, such as next-generation sequencing (NGS), have made carrier screening more accessible, precise, and cost-effective, encouraging widespread adoption among individuals and couples seeking to assess the risk of passing on inherited conditions.
In addition, growing support from healthcare professionals, organizations, and government initiatives promoting genetic testing has contributed significantly to market expansion. The increasing shift toward personalized healthcare, with more individuals opting for genetic counseling to make informed reproductive choices, is also boosting the trend. Furthermore, the high prevalence of genetic disorders and the growing focus on preventive healthcare are accelerating the adoption of carrier screening services. These factors collectively drive the continued growth of the North American carrier screening market, positioning it for sustained expansion in the future.
Report Scope and North America Carrier Screening Market Segmentation
|
Attributes |
North America Carrier Screening Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Color Health, Inc. (U.S.), Danaher Corporation (U.S.), Fulgent Genetics. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Helix, Inc. (U.S.), Illumina, Inc. (U.S.), Karius, Inc. (U.S.), Labcorp Genetics Inc. (U.S.), Laboratory Corporation of America Holdings (U.S.), MedGenome (U.S.), Myriad Genetics, Inc. (U.S.), Natera, Inc. (U.S.), Otogenetics (U.S.), OPKO Health, Inc. (U.S.), PerkinElmer (U.S.), Quest Diagnostics Incorporated (U.S.), Synbio Technologies (U.S.), Synthego (U.S.), Sequencing Inc. (U.S.) and Thermo Fisher Scientific Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Carrier Screening Market Definition
Carrier screening refers to a type of genetic test used to determine if an individual carries a gene for a recessive inherited genetic disorder. It helps identify people who may be at risk of passing genetic conditions, such as cystic fibrosis, sickle cell anemia, or Tay-Sachs disease, to their offspring. Carrier screening is commonly performed before or during pregnancy, allowing individuals or couples to make informed reproductive decisions. With advancements in genetic testing technologies, including next-generation sequencing (NGS), the process has become more accurate, comprehensive, and widely accessible in North America. The growing emphasis on preventive healthcare and personalized medicine is driving the increasing adoption of carrier screening in the region.
Carrier Screening Market Dynamics
Drivers
- Technological Advancements in Genetic Testing
Technological advancements in genetic testing, particularly the development of next-generation sequencing (NGS), is a major driver of the North America carrier screening market. NGS allows for faster, more accurate, and comprehensive genetic analysis, enabling the screening of a wide range of genetic conditions in a single test. For instance, companies such as Counsyl (now part of Myriad Genetics) have leveraged NGS technology to offer expanded carrier screening panels that test for hundreds of inherited conditions, making it more efficient and cost-effective. The increased accuracy and reduced cost of these tests have made carrier screening more accessible to a broader population, encouraging early detection of genetic risks. This technological progression allows healthcare providers to offer more personalized and preventative care, driving the demand for carrier screening services across North America.
- Growing Awareness and Acceptance of Genetic Testing
Increasing public awareness about genetic disorders and the benefits of early genetic screening has significantly driven the North American market. As more people understand the importance of identifying carrier status before or during pregnancy, they are more inclined to seek testing. For instance, organizations such as the American College of Obstetricians and Gynecologists (ACOG) have endorsed carrier screening for certain genetic conditions, leading to greater acceptance and demand. This shift in awareness is also supported by advocacy groups that promote the importance of genetic counseling and screening, such as the Genetic Alliance. As the awareness of genetic risks grows, more individuals and couples are opting for carrier screening, contributing to the market's expansion. The increased focus on preventive healthcare and informed reproductive decision-making is accelerating the uptake of carrier screening across North America.
Opportunities
- Expansion of Expanded Carrier Screening Panels
A significant opportunity for the North America carrier screening market lies in the expansion of comprehensive, expanded carrier screening panels that test for a broader range of genetic conditions. These tests provide valuable information to individuals and couples about their genetic risks, even for rare or less common disorders. For instance, companies such as Invitae and Fulgent Genetics have developed expanded panels that test for hundreds of conditions, addressing a wider variety of genetic risks than traditional screenings. As the demand for personalized healthcare and prevention grows, individuals are increasingly seeking these comprehensive screenings to gain a more thorough understanding of their genetic health. The growing trend toward early detection and prevention presents a substantial market opportunity, particularly in providing more inclusive and accessible genetic testing options for the general population.
- Integration with Prenatal and Reproductive Health Services
There is a significant opportunity for the carrier screening market through its integration with broader prenatal and reproductive health services. As more healthcare providers incorporate carrier screening into routine preconception and prenatal care, it can become a standard part of reproductive health management. For instance, many fertility clinics and obstetricians are now recommending carrier screening as part of preconception counseling. By integrating genetic testing with services such as IVF (in vitro fertilization) and genetic counseling, clinics can offer more tailored care for individuals and couples considering family planning options. This integration helps reduce the risk of passing on genetic disorders, leading to better-informed reproductive decisions. As awareness and acceptance of genetic testing grow, healthcare providers are increasingly adopting these services, presenting an opportunity for market growth in the context of comprehensive reproductive health strategies.
Restraints/Challenges
- High Costs of Genetic Testing
One of the primary restraints in the North America carrier screening market is the high cost of genetic testing, which can limit access for some individuals. While advancements in technology have made testing more affordable, many tests, especially expanded panels, remain expensive. For instance, tests offered by companies such as Myriad Genetics or Invitae can cost several hundred to thousands of dollars, and although insurance may cover some of these costs, many individuals face out-of-pocket expenses. This can be a barrier for people without comprehensive insurance or those who may not be able to afford the additional financial burden. While costs are expected to decrease over time, the current financial barrier may slow the widespread adoption of carrier screening, particularly among underinsured or economically disadvantaged populations, limiting the market's potential growth.
- Regulatory and Ethical Concerns
A significant challenge facing the North America carrier screening market involves regulatory and ethical concerns surrounding genetic testing. As genetic screening becomes more widespread, questions around privacy, data security, and how genetic information is used by insurance companies or employers have raised concerns. For instance, the Genetic Information Nondiscrimination Act (GINA) in the U.S. was introduced to prevent discrimination based on genetic information, but there are still fears among patients about the potential misuse of their genetic data. In addition, there are ethical dilemmas related to the disclosure of genetic risks, especially in the context of prenatal screening, where decisions about pregnancy outcomes may be influenced by the results. These regulatory and ethical complexities can hinder the growth of the market, as both consumers and healthcare providers navigate the potential risks and legal implications of genetic testing.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Carrier Screening Market Scope
The market is segmented on the basis of type of screening, technology, medical conditions and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type of Screening
- Expanded Carrier Screening
- Targeted Disease Carrier Screening
Technology
- DNA Sequencing
- Polymerase Chain Reaction
- Microarrays
- Others
Medical Conditions
- Spinal Muscular Atrophy
- Cystic Fibrosis
- Tay-Sachs
- Gaucher Disease
- Sickle Cell Disease
- Other Medical Conditions
End User
- Hospitals & Clinics
- Reference Laboratories
- Physician Offices
- Others
North America Carrier Screening Market Regional Analysis
The market is analysed and market size insights and trends are provided by type of screening, technology, medical conditions and end user as referenced above.
The countries covered in the market report are U.S., Canada, Mexico in North America, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), Brazil, Argentina, Rest of South America as a part of South America, U.A.E, Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA)
The U.S. is expected to dominate the North America carrier screening market. This is primarily due to the country's advanced healthcare infrastructure, high adoption rate of genetic testing technologies, and significant investment in genetic research and innovation. The U.S. also benefits from a large and diverse population, which drives demand for comprehensive genetic testing, particularly for individuals seeking personalized healthcare and reproductive decision-making options. In addition, the widespread availability of genetic counseling services and the growing focus on preventive healthcare contribute to the U.S.'s leadership in the market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
North America Carrier Screening Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
North America Carrier Screening Market Leaders Operating in the Market Are:
- Color Health, Inc. (U.S.)
- Danaher Corporation (U.S.)
- Fulgent Genetics. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Helix, Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Karius, Inc. (U.S.)
- Labcorp Genetics Inc. (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- MedGenome (U.S.)
- Myriad Genetics, Inc. (U.S.)
- Natera, Inc. (U.S.)
- Otogenetics (U.S.)
- OPKO Health, Inc. (U.S.)
- PerkinElmer (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Synbio Technologies (U.S.)
- Synthego (U.S.)
- Sequencing Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
Latest Developments in North America Carrier Screening Market
- In November 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a label expansion for the PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody test to include biliary tract cancer (BTC). This makes it the first and only FDA-approved companion diagnostic to assess HER2-positive status, helping identify BTC patients who are eligible for treatment with Jazz Pharmaceuticals' ZIIHERA.
- In June 2024, Illumina Inc. introduced DRAGEN v4.3, the newest version of its DRAGEN software, which is part of the Illumina Connected Software portfolio for analyzing next-generation sequencing data. DRAGEN v4.3 incorporates a range of cutting-edge innovations that enable a more comprehensive genome analysis, including support for next-generation multigenome with 128 samples, machine learning mosaic mode, and additional features.
- In July 2024, Danaher Corporation unveiled the opening of two new CLIA and CAP-certified laboratories designed to expedite the development of Companion Diagnostics (CDx) and Complementary Diagnostics (CoDx). These diagnostic tests help ensure patients receive targeted therapies, enhancing the effectiveness of their disease treatment.
- In March 2024, Centogene N.V. announced the expansion of its ongoing partnership with Takeda to improve the diagnosis of patients with Lysosomal Storage Disorders (LSDs). The extended agreement aims to increase patient access to fast and accurate diagnostics for LSDs, including Fabry disease, Gaucher disease, and Hunter syndrome.
- In February 2024, Danaher Corporation entered into a strategic partnership with Cincinnati Children's Hospital Medical Center, aiming to enhance patient safety by tackling a major cause of failure in clinical trials. This collaboration, part of the Danaher Beacons program, focuses on advancing liver organoid technology as a drug toxicity screening solution to protect patients. The initiative has the potential to accelerate the development of new therapies and could save billions of dollars annually in lost research and development productivity.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.