North America Dermatology Diagnostic Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
1.20 Billion
USD
2.40 Billion
2024
2032
USD
1.20 Billion
USD
2.40 Billion
2024
2032
| 2025 –2032 | |
| USD 1.20 Billion | |
| USD 2.40 Billion | |
|
|
|
|
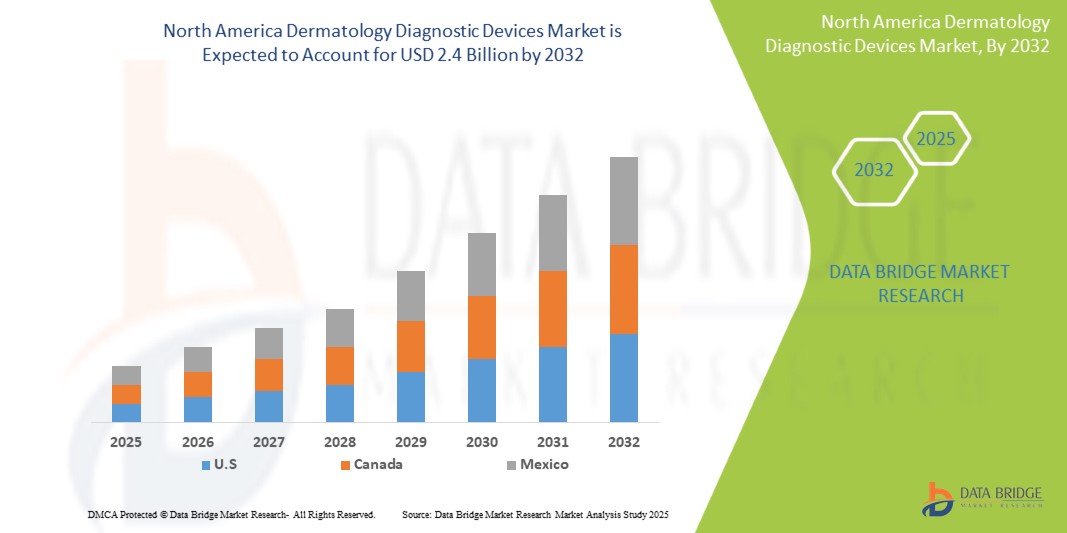
North America Dermatology Diagnostic Devices Market Size
- The North America Dermatology Diagnostic Devices Market was valued at USD 1.2 Billion in 2024 and is expected to reach USD 2.4 Billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 9.2%, primarily driven by the Growing Demand for Minimally Invasive Cosmetic Procedures
- Key drivers of the North America Dermatology Diagnostic Devices Market include the growing demand for minimally invasive cosmetic procedures, increased preference for natural-looking results, and advancements in fat processing technologies.
North America Dermatology Diagnostic Devices Market Analysis
- Autologous fat grafting is becoming increasingly popular due to its minimally invasive nature, providing natural-looking results with less risk and downtime compared to traditional surgical methods.
- Cosmetic and Reconstructive Applications: The market is driven by growing demand for cosmetic procedures like breast and buttock augmentation, facial rejuvenation, and hand rejuvenation, as well as its use in reconstructive surgeries following trauma or cancer treatments.
- Technological Advancements: Innovations in fat processing and harvesting techniques have improved the efficiency, safety, and effectiveness of fat grafting procedures, making them more reliable and accessible to patients.
- For instance, Increasing awareness and acceptance of cosmetic procedures, particularly among aging populations and individuals seeking body contouring, are significantly contributing to market growth.
- The market is witnessing significant growth in emerging economies, driven by rising disposable incomes, advancements in healthcare infrastructure, and a growing middle-class population seeking cosmetic enhancements.
Report Scope and North America Dermatology Diagnostic Devices Market Segmentation
|
Attributes |
North America Dermatology Diagnostic Devices Market Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Dermatology Diagnostic Devices Market Trends
“shift towards regenerative treatments and natural-looking results”
- Regenerative Alternatives to Synthetic Fillers: Injectables like Renuva, derived from donated human adipose tissue, are gaining popularity as they stimulate the body's own fat production, offering a more natural and sustainable solution compared to traditional synthetic fillers.
- Celebrity Influence and Social Media Normalization: High-profile figures openly discussing their cosmetic enhancements on platforms such as Instagram and Twitter have reduced the stigma surrounding cosmetic interventions, leading to increased societal acceptance and rising consumer demand for fat grafting procedures.
North America Dermatology Diagnostic Devices Market Dynamics
Driver
“Increasing Demand for Minimally Invasive Cosmetic Procedures”
- Preference for Natural Results: Patients are increasingly seeking cosmetic procedures that offer natural-looking outcomes with minimal scarring and recovery time. Autologous fat grafting, which uses the patient's own fat, aligns with this demand by providing subtle enhancements without the risks associated with synthetic implants.
- Advancements in Fat Processing Technologies: Innovations in fat harvesting, purification, and injection methods have enhanced the safety and efficacy of autologous fat grafting. These technological advancements have improved graft retention rates and minimized complications, making the procedure more appealing to both patients and healthcare providers.
- Growing Acceptance of Aesthetic Procedures: Cultural shifts and increased awareness have led to a broader acceptance of aesthetic enhancements. As societal attitudes evolve, more individuals are opting for procedures like autologous fat grafting to achieve desired cosmetic results, contributing to the market's growth.
Opportunity
“Integration of Regenerative Injectables like Renuva”
- Natural-Looking Results: Renuva offers a more natural and sustainable solution compared to traditional synthetic fillers by gradually restoring the body's own fat, reducing the risk of complications associated with synthetic materials.
- Minimized Risk of Overcorrection: Unlike traditional fillers that can lead to overcorrection, Renuva's gradual integration into the body allows for more controlled and subtle enhancements, appealing to patients seeking natural results.
- Complementary to Fat Grafting: Renuva can be used in conjunction with traditional fat grafting procedures to enhance outcomes, especially in patients with limited fat availability, thereby expanding the applicability of fat grafting techniques.
Restraint/Challenge
“High Costs and Post-Procedure Complications”
- Autologous fat grafting procedures can be expensive, with costs ranging from $3,000 to $7,500 for facial fat grafting in regions like California. This financial burden may deter potential patients, especially in developing economies where disposable income is lower.
- While generally safe, autologous fat grafting carries risks such as fat necrosis, infection, and uneven results. These complications can lead to additional medical expenses and may deter potential patients.
- In many regions, autologous fat grafting procedures are considered elective and are not covered by insurance. This lack of reimbursement options further increases the financial burden on patients, limiting accessibility to the procedure.
North America Dermatology Diagnostic Devices Market Scope
The market is segmented on the basis application, product type, gender, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Application |
|
|
By Product Type |
|
|
By gender |
|
|
By End User
|
|
|
By Distribution Channel
|
|
North America Dermatology Diagnostic Devices Market Regional Analysis
“North America is the Dominant Region in the Global Autologous Fat Grafting Market”
- High Market Share: North America accounts for over 50% of the North America Dermatology Diagnostic Devices Market share, driven by technological advancements and increasing research and development activities in the region.
- Advanced Healthcare Infrastructure: The region boasts a well-established medical technology industry and robust R&D activities, supporting the adoption of innovative fat grafting techniques.
- Strong Demand for Cosmetic Procedures: There is a high demand for cosmetic procedures, including breast augmentation and facial rejuvenation, contributing to the market's growth.
- Regulatory Approvals and Innovation: The expansion of regulatory approvals for innovative fat grafting products fosters market growth and encourages innovation in treatment options.
“Asia-Pacific is Projected to Register the Highest Growth Rate in the Global Autologous Fat Grafting Market”
- Increasing Disposable Incomes: Rising disposable incomes in countries like China, India, and Japan enable more individuals to afford cosmetic procedures, thereby expanding the patient base for autologous fat grafting.
- Growing Medical Tourism: Countries such as Thailand, Malaysia, and South Korea are becoming popular destinations for medical tourism, attracting international patients seeking affordable and high-quality cosmetic treatments.
- Cultural Acceptance of Aesthetic Procedures: There is a growing cultural acceptance of cosmetic enhancements in the Asia-Pacific region, leading to increased demand for procedures like autologous fat grafting.
North America Dermatology Diagnostic Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Allergan plc
- Cytori Therapeutics, Inc.
- Sisram Medical Ltd (Alma Lasers)
- Genesis Biosystems, Inc.
- Black Tie Medical Inc. (Tulip Medical Inc.)
- Ranfac Corp.
- HK Surgical Inc.
- Human Med AG
- MicroAire Surgical Instruments LLC
- Sterimedix Ltd.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.