North America Electronic Clinical Outcome Assessment Ecoa Market
Market Size in USD Million
CAGR :
% 
 USD
265.11 Million
USD
924.58 Million
2024
2032
USD
265.11 Million
USD
924.58 Million
2024
2032
| 2025 –2032 | |
| USD 265.11 Million | |
| USD 924.58 Million | |
|
|
|
|
North America Electronic Clinical Outcome Assessment (eCOA) Market Size
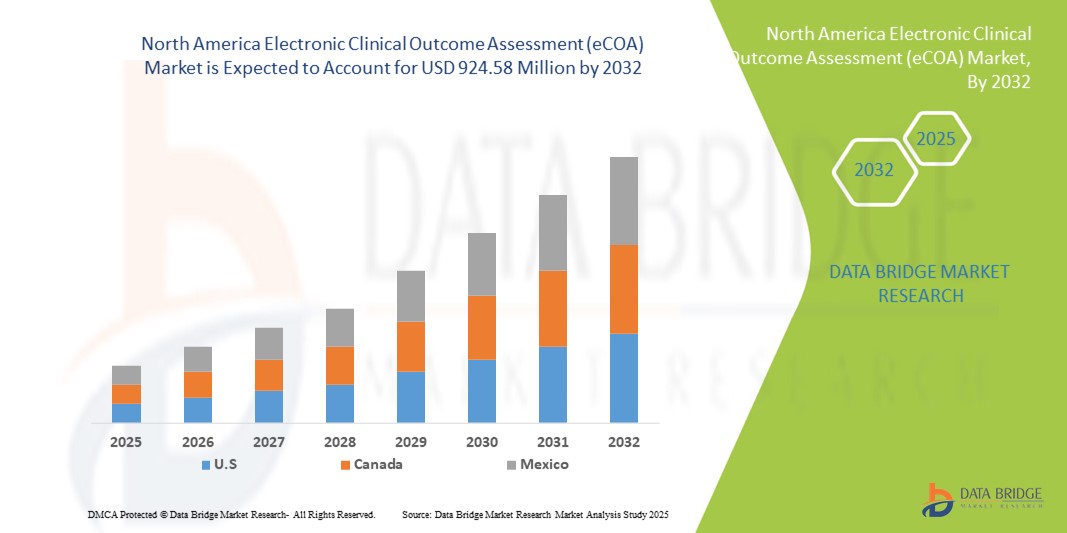
- The North America electronic clinical outcome assessment (eCOA) market size was valued at USD 265.11 million in 2024 and is expected to reach USD 924.58 million by 2032, at a CAGR of 16.90% during the forecast period
- The market growth is largely fueled by the increasing digitization of clinical trials and the rising adoption of technology-driven healthcare solutions, leading to enhanced efficiency, accuracy, and patient-centric approaches in clinical studies
- Furthermore, the growing need for real-time patient data capture, remote monitoring, and regulatory compliance is driving the adoption of Electronic Clinical Outcome Assessment (eCOA) solutions, thereby significantly boosting the industry's growth. These converging factors are enabling pharmaceutical and biotechnology companies, as well as contract research organizations, to streamline trial operations and improve overall clinical outcomes
North America Electronic Clinical Outcome Assessment (eCOA) Market Analysis
- Electronic Clinical Outcome Assessment (eCOA) solutions are increasingly becoming essential components in clinical trials, offering real-time, accurate, and patient-centered data collection to improve study quality, regulatory compliance, and operational efficiency
- The escalating adoption of eCOA is primarily fueled by the growing demand for decentralized and remote clinical trials, increasing focus on patient-centric research, and the need for efficient data capture across multiple sites
- U.S. dominated the electronic clinical outcome assessment (eCOA) market with the largest revenue share of 79.01% in 2024, driven by the presence of major pharmaceutical and biotechnology companies, advanced clinical research infrastructure, and strong investment in digital health solutions. The U.S., in particular, experienced substantial growth in eCOA implementations, supported by large-scale clinical trial pipelines, government initiatives encouraging digital adoption, and a robust technology ecosystem that facilitates integration with electronic data capture and trial management systems
- Canada is expected to be the fastest-growing country in the North American electronic clinical outcome assessment (eCOA) market during the forecast period, due to increasing government support for digital clinical solutions, rising outsourcing of clinical trials, and growing adoption of cloud-based and mobile eCOA platforms. Expansion of research networks and rising awareness among healthcare providers regarding patient-centric technologies further accelerate market growth in the country
- The patient reported outcome assessment (PRO) segment dominated the electronic clinical outcome assessment (eCOA) market with the largest market revenue share of 52.1% in 2024, as patient-centric trials become increasingly important
Report Scope and Electronic Clinical Outcome Assessment (eCOA) Market Segmentation
|
Attributes |
Electronic Clinical Outcome Assessment (eCOA) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Electronic Clinical Outcome Assessment (eCOA) Market Trends
Increasing Adoption of Digital Patient-Reported Outcomes and Remote Monitoring
- A significant and accelerating trend in the United States Electronic Clinical Outcome Assessment (eCOA) market is the growing adoption of digital patient-reported outcome (ePRO) platforms and remote data capture solutions. These technologies are improving data accuracy, real-time monitoring, and patient engagement across clinical trials
- For instance, the implementation of eCOA solutions in multi-site Phase III oncology trials has enabled seamless collection of patient symptom data, reducing manual entry errors and improving compliance with protocol-defined assessments
- Remote monitoring platforms allow clinical staff to track patient-reported outcomes in real time, enabling faster intervention, enhanced trial oversight, and more reliable data for regulatory submissions
- The integration of cloud-based eCOA systems with clinical trial management platforms ensures that collected data can be efficiently accessed, aggregated, and analyzed across multiple trial sites, supporting centralized study management
- There is a growing emphasis on patient-centric trial designs, where eCOA solutions enhance convenience and adherence, leading to higher data completeness and quality
- Regulatory agencies and sponsors are increasingly recognizing the value of electronic outcome assessments in improving trial efficiency and accelerating decision-making, encouraging further adoption of these solutions
North America Electronic Clinical Outcome Assessment (eCOA) Market Dynamics
Driver
Growing Need Due to Rising Demand for Digital Clinical Data Capture and Patient-Centric Trials
- The increasing emphasis on accurate, real-time clinical data collection, coupled with the growing shift towards patient-centric trial designs, is a significant driver for the heightened demand for eCOA solutions
- For instance, in April 2024, Medidata Solutions announced the launch of an enhanced electronic patient-reported outcome platform designed to integrate seamlessly with global clinical trial management systems. Such advancements by key companies are expected to drive the eCOA industry growth in the forecast period
- As sponsors and clinical research organizations aim to improve trial efficiency and compliance, eCOA platforms offer features such as remote data capture, automated notifications, and real-time monitoring of patient-reported outcomes, providing a compelling alternative to traditional paper-based methods
- Furthermore, the growing adoption of decentralized and virtual clinical trials is making eCOA systems an integral component of modern study designs, enabling participants to report outcomes from home or local healthcare facilities
- The convenience of remote monitoring, integration with clinical trial analytics platforms, and improved regulatory compliance are key factors propelling the adoption of eCOA solutions across pharmaceutical, biotechnology, and contract research organizations. The trend towards digital transformation in clinical trials further contributes to market growth
Restraint/Challenge
Concerns Regarding Data Security, Regulatory Compliance, and High Initial Implementation Costs
- Concerns surrounding data privacy, cybersecurity, and compliance with regulations such as HIPAA and GDPR pose a significant challenge to broader market penetration. eCOA systems handle sensitive patient information, and breaches or non-compliance could lead to substantial financial and legal consequences
- For instance, stringent regulatory audits and increasing scrutiny on digital clinical data have made some sponsors cautious about adopting new eCOA platforms without validated security protocols
- Addressing these concerns through robust encryption, secure authentication methods, and adherence to global regulatory standards is crucial for building trust among sponsors, CROs, and clinical sites. Companies such as ERT and CRF Health emphasize their secure platforms and compliance certifications to reassure stakeholders
- In addition, the relatively high initial cost of comprehensive eCOA implementations can be a barrier for smaller biopharmaceutical companies or early-stage clinical trials
- While subscription models and cloud-based platforms are gradually reducing upfront costs, the perceived premium for fully integrated eCOA solutions can still hinder adoption, particularly in emerging markets or for budget-conscious organizations
- Overcoming these challenges through enhanced data security measures, training programs for clinical staff, and development of scalable, cost-effective eCOA options will be vital for sustained market growth
North America Electronic Clinical Outcome Assessment (eCOA) Market Scope
The market is segmented on the basis of product, approach, end user, and platform.
- By Product
On the basis of product, the electronic clinical outcome assessment (eCOA) market is segmented into on-premise solutions, cloud-based solutions, and web-based solutions. The cloud-based solutions segment dominated the market with the largest revenue share of 47.5% in 2024, driven by its scalability and flexibility in managing complex clinical trials. Cloud solutions provide real-time access to data across multiple trial sites, enabling faster decision-making and improved operational efficiency. The ability to integrate mobile devices and ensure secure data storage has increased adoption among pharmaceutical companies and CROs. Cloud platforms reduce IT infrastructure costs and support decentralized and hybrid trial models. Their user-friendly interfaces and automated reporting tools further enhance data accuracy. Remote monitoring and centralized control make them ideal for large-scale, multi-site studies. The segment benefits from continuous innovation and vendor support, maintaining its dominance in the market.
The on-premise solutions segment is expected to witness the fastest CAGR of 18.2% from 2025 to 2032, as organizations prioritize internal data control and security. On-premise platforms provide robust compliance with regulatory requirements, allowing full control over sensitive patient and trial data. They can be customized to meet specialized workflow needs and integrated with existing hospital or institutional IT infrastructure. On-premise solutions are particularly favored for trials requiring high-security measures, such as oncology or rare disease studies. Rising investment in hospital and research center IT capabilities is driving adoption. These systems offer enhanced data privacy and operational continuity during internet outages. Continuous software updates and technical support further enhance reliability. The increasing shift toward digital clinical trials supports the growing uptake of on-premise platforms.
- By Approach
On the basis of approach, the electronic clinical outcome assessment (eCOA) market is segmented into Clinician Reported Outcome Assessment (CLINRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (OBSRO), and Performance Outcome Assessment (PERFO). The Patient Reported Outcome Assessment (PRO) segment held the largest market revenue share of 52.1% in 2024, as patient-centric trials become increasingly important. PRO solutions enable participants to report symptoms, quality of life metrics, and treatment responses directly, improving data accuracy and engagement. Regulatory authorities recognize PRO data as critical for drug approval and post-marketing surveillance. Integration with mobile apps allows reminders, e-diaries, and automated reporting. PRO assessments enhance real-time monitoring and early detection of adverse events. They reduce dependency on manual data collection and improve trial efficiency. Sponsors benefit from actionable insights for adaptive trial designs. The segment’s flexibility and global standardization contribute to its continued dominance.
The clinician reported outcome assessment (CLINRO) segment is expected to witness the fastest CAGR of 16.4% from 2025 to 2032, driven by growing adoption in hospital-based studies and complex clinical research. CLINRO tools allow clinicians to provide structured evaluations of patient outcomes, ensuring high-quality data for trials involving complex interventions. These assessments are crucial for regulatory submissions and validation of therapeutic efficacy. Integration with electronic health records and clinical trial management systems improves workflow efficiency. Digital CLINRO platforms reduce errors, streamline documentation, and enable remote data entry. Increasing adoption in oncology, cardiology, and neurology studies is contributing to growth. Enhanced reporting features and analytics provide deeper insights into treatment effectiveness. The segment benefits from expanding training programs and regulatory support, accelerating uptake.
- By End User
On the basis of end user, the electronic clinical outcome assessment (eCOA) market is segmented into commercial service providers, hospitals and transplant centers, and research laboratories and academic institutions. The hospitals and transplant centers segment dominated the market with the largest revenue share of 49.3% in 2024, due to the high volume of clinical trials conducted in these facilities. Hospitals benefit from integrated IT infrastructure, trained staff, and multi-site trial capabilities. eCOA solutions enhance patient engagement, reduce manual data entry, and ensure compliance with regulatory standards. Remote monitoring and automated reporting improve operational efficiency. Hospitals can leverage real-time insights to optimize study protocols. Growing adoption of hybrid and decentralized trials further supports demand. Integration with electronic medical records ensures seamless workflow. Advanced analytics within hospital systems aids in faster decision-making and resource optimization.
The research laboratories and academic institutions segment is expected to witness the fastest CAGR of 17.1% from 2025 to 2032, as these entities increasingly adopt digital solutions to improve study efficiency. Academic institutions conduct diverse clinical trials and collaborative research requiring accurate and timely data collection. Cloud and on-premise eCOA platforms enable seamless coordination across multiple research sites. Funding from government and private sources is driving technology investments. eCOA adoption allows faster enrollment, better participant engagement, and efficient data management. Advanced reporting and analytics enhance research insights. Institutions benefit from scalable solutions supporting various trial designs. Growing partnerships between academia and industry contribute to rapid uptake. Digital literacy among researchers facilitates adoption and integration into study workflows.
- By Platform
On the basis of platform, the electronic clinical outcome assessment (eCOA) market is segmented into Contract Research Organizations, Pharmaceutical and Biopharmaceutical Companies, Medical Device Manufacturers, Hospitals and Clinical Laboratories, Consulting Service Companies, and Research and Academia. The Pharmaceutical and Biopharmaceutical Companies segment held the largest market revenue share of 50.2% in 2024, driven by their pivotal role in global clinical trials. These companies require accurate patient outcome tracking for regulatory submissions and drug approvals. eCOA platforms provide real-time monitoring, secure data storage, and integration with trial management systems. Adoption is supported by large R&D budgets and global trial operations. Cloud and hybrid solutions enable multi-site coordination and adaptive trial designs. Automated reporting enhances efficiency and minimizes human error. Regulatory compliance and audit readiness are ensured through secure digital systems. The increasing focus on patient-centric drug development sustains dominance in this segment.
The Contract Research Organizations (CROs) segment is expected to witness the fastest CAGR of 18.5% from 2025 to 2032, fueled by the outsourcing of clinical trial management by pharmaceutical sponsors. CROs leverage eCOA platforms to manage complex studies across multiple sites efficiently. Cloud-based and mobile-enabled solutions facilitate real-time data capture and monitoring. Adoption is accelerated by decentralized and hybrid trial models. eCOA integration improves patient engagement, reduces operational bottlenecks, and ensures regulatory compliance. CROs increasingly invest in advanced analytics and reporting tools to support clients’ decision-making. Training and standardized workflows further enhance efficiency. Expansion of global trial networks drives adoption in North America. Continuous platform upgrades and service innovations sustain rapid growth.
North America Electronic Clinical Outcome Assessment (eCOA) Market Regional Analysis
- North America dominated the electronic clinical outcome assessment (eCOA) market with the largest revenue share in 2024, driven by the presence of major pharmaceutical and biotechnology companies, advanced clinical research infrastructure, and strong investment in digital health solutions. The region has witnessed rapid adoption of digital clinical platforms, particularly in large-scale and multi-site trials, supporting the demand for real-time data capture and streamlined trial management
- The widespread adoption of eCOA in North America is further bolstered by a robust technology ecosystem that enables seamless integration with electronic data capture systems, clinical trial management platforms, and analytics solutions. Sponsors and CROs benefit from enhanced operational efficiency, regulatory compliance, and improved patient engagement through these integrated digital platforms
- High research funding, government initiatives promoting digital health adoption, and the presence of key technology providers in the region further reinforce North America’s leading position in the global eCOA market
U.S. Electronic Clinical Outcome Assessment (eCOA) Market Insight
The U.S. electronic clinical outcome assessment (eCOA) market dominated the North America market within North America with the largest revenue share of 79.01% in 2024, fueled by the rapid adoption of digital clinical platforms, large-scale clinical trial pipelines, and government initiatives supporting technological integration in healthcare. The extensive presence of pharmaceutical and biotechnology companies drives substantial demand for eCOA solutions to manage complex, multi-site trials efficiently. The U.S. market benefits from advanced clinical research infrastructure, ensuring accurate and compliant patient data capture. Increasing investment in digital health solutions, the integration of eCOA with electronic data capture (EDC) systems, and the need for real-time monitoring of patient-reported outcomes further propel market growth. Healthcare providers and trial sponsors in the U.S. are increasingly prioritizing patient-centric technologies, which facilitate improved trial engagement, adherence, and reporting accuracy, reinforcing the country’s dominant market position.
Canada Electronic Clinical Outcome Assessment (eCOA) Market Insight
The Canada electronic clinical outcome assessment (eCOA) market is expected to be the fastest-growing country in the North American market during the forecast period, driven by increasing government support for digital clinical solutions and rising outsourcing of clinical trials to specialized service providers. The growing adoption of cloud-based and mobile eCOA platforms enables efficient patient data collection, improved regulatory compliance, and enhanced flexibility for trial sponsors across the country. Expansion of research networks, rising awareness among healthcare providers regarding patient-centric trial technologies, and the need for more streamlined data capture in multi-site trials accelerate eCOA market growth in Canada. Increasing investment in digital infrastructure and supportive policies further bolster Canada’s trajectory as the fastest-growing market for eCOA solutions in North America.
North America Electronic Clinical Outcome Assessment (eCOA) Market Share
The electronic clinical outcome assessment (eCOA) industry is primarily led by well-established companies, including:
- IQVIA (U.S.)
- Clario (U.S.)
- Medidata (U.S.)
- Veeva Systems (U.S.)
- Earth Resources Technology (U.S.)
- Oracle Health Sciences (U.S.)
- YPrime, LLC (U.S.)
- ArisGlobal LLC (U.S.)
- Castor EDC (Netherlands)
- eClinicalWorks (U.S.)
- Medrio, Inc. (U.S.)
- ClinOne (U.S.)
- Signant Health (U.S.)
- Clinical Ink, Inc. (U.S.)
- Curebase, Inc. (U.S.)
- Kayentis (France)
- Calyx (U.K.)
- Datacubed Health (U.S.)
- HealthDiary, Inc. (U.S.)
Latest Developments in North America Electronic Clinical Outcome Assessment (eCOA) Market
- In December 2023, ObvioHealth USA, Inc., a global digital clinical trials company, launched an electronic clinical outcome assessment (eCOA) solution. This platform seamlessly integrates advanced study design technology with scientific and clinical services to deliver more robust outcomes for trial sponsors
- In May 2025, Medidata, a prominent player in the eCOA space, was recognized as a leader in Everest Group's PEAK Matrix Assessment for eCOA. The evaluation highlighted Medidata's support for over 1 million patients and its ability to reduce study build timelines by up to 50% compared to the industry standard
- In March 2025, the Critical Path Institute (C-Path) announced the successful conclusion of the eCOA: Getting Better Together Initiative. This collaboration, driven by a shared commitment to advancing patient-focused drug development, resulted in meaningful, lasting changes benefiting all stakeholders across the eCOA ecosystem
- In June 2025, Medable Inc., a global provider of clinical development technology, introduced its new Partner Program. This initiative aims to empower contract research organizations (CROs) and other partners with generative AI-based, self-service eCOA build capabilities for digitally enabled clinical trials
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.