North America Hepatitis B Infection Market
Market Size in USD Billion
CAGR :
% 
 USD
2.46 Billion
USD
3.61 Billion
2024
2032
USD
2.46 Billion
USD
3.61 Billion
2024
2032
| 2025 –2032 | |
| USD 2.46 Billion | |
| USD 3.61 Billion | |
|
|
|
|
North America Hepatitis B Infection Market Size
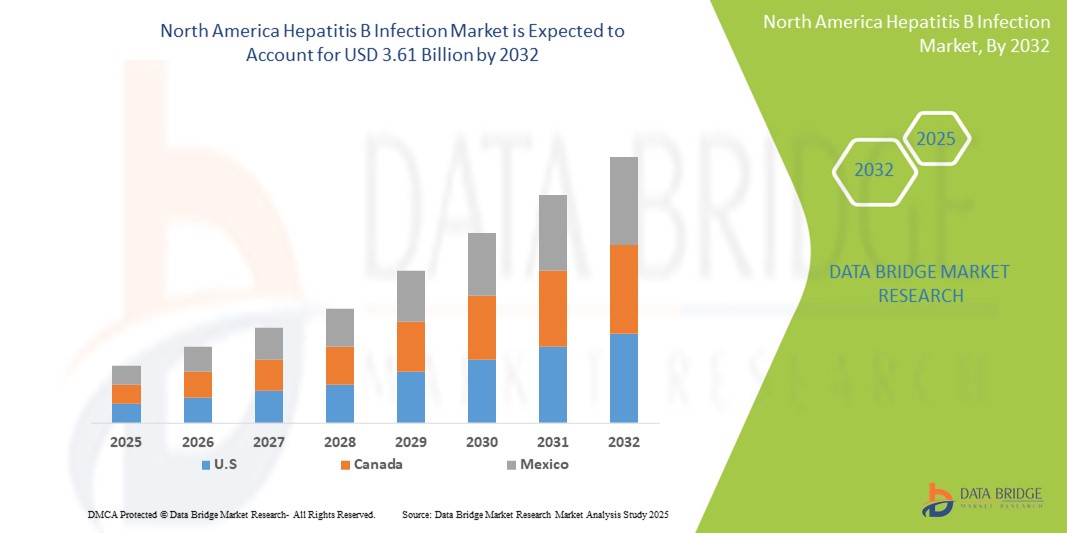
- The North America hepatitis B infection market size was valued at USD 2.46 billion in 2024 and is expected to reach USD 3.61 billion by 2032, at a CAGR of 4.90% during the forecast period
- The market growth is largely fueled by the growing adoption of advanced diagnostic technologies and therapeutic innovations for Hepatitis B, coupled with increasing digitalization and integration of electronic health systems across Europe
- Furthermore, rising consumer and public health demand for accurate, accessible, and preventive solutions is establishing Hepatitis B management protocols as a central focus of healthcare policy. These converging factors are accelerating the adoption of vaccination, screening, and antiviral therapies, thereby significantly boosting the Hepatitis B Infection market growth across the region
North America Hepatitis B Infection Market Analysis
- Hepatitis B treatments and diagnostics are increasingly vital components of North America’s public health infrastructure, especially in both hospital and outpatient settings, due to rising infection awareness, improved testing accessibility, and advancements in antiviral therapies
- The escalating demand for effective Hepatitis B management is primarily fueled by widespread immunization efforts, increased HBV-HDV co-infection screening, and the rising burden of chronic liver diseases—particularly among aging populations and immigrant communities
- U.S. dominated the North America hepatitis B infection market with the largest revenue share of 57.9% in 2024, characterized by strong public health policies, the early adoption of advanced diagnostic technologies, and high HBV testing rates. The country has also witnessed significant growth in treatment uptake, especially among high-risk groups, fueled by public awareness campaigns, state-level elimination plans, and expanded insurance coverage for hepatitis care
- Mexico is expected to be the fastest-growing country within the North America hepatitis B infection market during the forecast period, supported by increasing urbanization, improved vaccination coverage, expanding government funding for hepatitis elimination, and enhanced access to healthcare services across both urban and rural areas
- The chronic hepatitis B segment dominated the North America hepatitis B infection market with a market share of 62.4% in 2024, owing to the persistent nature of the disease, the need for long-term antiviral therapy, and improved detection through targeted screening programs and integration of hepatitis testing into primary care
Report Scope and North America Hepatitis B Infection Market Segmentation
|
Attributes |
North America Hepatitis B Infection Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Hepatitis B Infection Market Trends
“Enhanced Convenience Through Integrated Care and Advanced Treatment Access”
- A significant and accelerating trend in the North America hepatitis B infection market is the growing integration of multidisciplinary care models and advanced treatment access through centralized healthcare systems. This trend is significantly improving patient outcomes and adherence by enabling seamless communication between general practitioners, hepatologists, and public health institutions
- For instance, several Western European countries have implemented national hepatitis action plans that allow patients to receive early diagnosis, antiviral treatment, and regular follow-up care under one coordinated framework. Germany’s integrated care model, for instance, enables efficient linkage from diagnosis to treatment, reducing disease progression rates
- Efforts such as centralized patient registries, digital health record systems, and streamlined referral pathways are optimizing hepatitis B infection management by enabling timely intervention and monitoring. These systems allow healthcare providers to track liver function, treatment response, and co-infections such as hepatitis D in real-time
- The integration of advanced diagnostics with routine primary care services facilitates early detection of both acute and chronic cases. This centralized approach, combined with affordable access to newer antiviral therapies, enhances both individual patient care and broader public health surveillance
- This trend toward more streamlined, coordinated, and technology-supported hepatitis B care is fundamentally reshaping expectations within national healthcare systems. As a result, many European governments are expanding access to viral hepatitis screening, particularly among vulnerable and high-risk populations such as migrants, intravenous drug users, and the elderly
- The demand for accessible, efficient, and integrated hepatitis B treatment models is rapidly growing across both public and private healthcare sectors, as stakeholders increasingly focus on long-term disease control and alignment with the World Health Organization’s 2030 hepatitis elimination goals
North America Hepatitis B Infection Market Dynamics
Driver
“Growing Need Due to Rising Disease Burden and Preventive Healthcare Adoption”
- The increasing prevalence of hepatitis B infections across Europe, along with heightened awareness about liver diseases, is significantly driving the demand for early diagnosis, vaccination, and treatment solutions
- For instance, in April 2024, GlaxoSmithKline plc (GSK) expanded its European hepatitis B vaccine supply through a strategic partnership with regional healthcare systems, aiming to improve immunization rates across high-risk populations. Such initiatives by key market players are expected to fuel growth in the North America Hepatitis B Infection market over the forecast period
- As public health authorities and consumers become more aware of the long-term complications associated with chronic hepatitis B—such as cirrhosis and liver cancer—the adoption of preventive strategies such as vaccination and early screening continues to rise
- Furthermore, the integration of hepatitis B testing into routine health check-ups and the growing popularity of point-of-care diagnostic technologies are making hepatitis B management more accessible and efficient across Europe
- The availability of effective vaccines, oral antiviral drugs, and the development of advanced immune modulators are enabling better disease control. Government funding, reimbursement policies, and WHO-led hepatitis elimination goals are also boosting adoption rates in both public and private healthcare settings
Restraint/Challenge
“Concerns Regarding Treatment Accessibility and High Cost of Advanced Therapies”
- Despite medical advancements, limited access to advanced antiviral therapies and immune modulators in certain parts of North America remains a challenge, particularly in Eastern and Southern North America where healthcare disparities persist
- For instance, studies published in early 2024 indicated that some EU member states still face shortages in hepatitis B vaccines and limited access to novel treatment regimens due to procurement and reimbursement issues
- Bridging this gap requires policy-level efforts to harmonize hepatitis B care standards across all European nations, particularly through EU-level funding support, price negotiations, and streamlined regulatory approvals
- Moreover, while first-line antiviral drugs are becoming more affordable, newer generation therapies with improved efficacy often come at a higher cost, potentially limiting their uptake among uninsured or low-income populations
- Public mistrust or vaccine hesitancy, especially in post-pandemic Europe, is another barrier that must be addressed through awareness campaigns and healthcare provider engagement
- Overcoming these challenges through expanded insurance coverage, public-private partnerships, and increased investment in regional healthcare infrastructure will be crucial to sustaining long-term growth in the North America Hepatitis B Infection market
North America Hepatitis B Infection Market Scope
The market is segmented on the basis of type, and treatment.
• By Type
On the basis of type, the North America hepatitis B infection market is segmented into chronic and acute. The chronic segment dominated the largest market revenue share of 62.4% in 2024, primarily due to the high prevalence of chronic HBV cases and the need for lifelong disease management through antiviral therapies and monitoring.
The acute segment is anticipated to witness the fastest growth rate with a CAGR of 6.4% from 2025 to 2032, fueled by enhanced early screening efforts, public health initiatives, and growing awareness leading to timely diagnosis and treatment.
• By Treatment
On the basis of treatment, the North America Hepatitis B Infection market is segmented into vaccine, antiviral drugs, immune modulator drugs, and surgery. The vaccine segment held the largest revenue share of 41.2% in 2024, supported by national vaccination drives, increased birth-dose immunization, and strong uptake among high-risk adult populations.
The antiviral drugs segment is projected to witness the fastest CAGR of 7.1% from 2025 to 2032, driven by the expanding chronic HBV patient pool, advancements in oral therapies, and favorable reimbursement policies.
North America Hepatitis B Infection Market Regional Analysis
- North America dominated the hepatitis B infection market with the largest revenue share of 33.27% in 2024, driven by robust public health infrastructure, widespread vaccination programs, and growing awareness about hepatitis B transmission and prevention
- The region is characterized by cutting-edge diagnostic technologies, well-established immunization protocols, and proactive government-led hepatitis surveillance initiatives
- This strong uptake of preventive and therapeutic strategies is further supported by extensive healthcare access, ongoing R&D funding, and a focused effort on early detection and disease management, solidifying North America’s role as a key contributor to the global Hepatitis B Infection market
U.S. Hepatitis B Infection Market Insight
The U.S. hepatitis B infection market dominated the North America market with the largest revenue share of 57.9% in 2024, attributed to its advanced healthcare infrastructure, widespread HBV vaccination coverage, and early adoption of screening and diagnostic protocols. Government efforts such as CDC-recommended universal screening guidelines, along with strong support for HBV-HDV co-infection testing, are driving market growth. In addition, large-scale awareness campaigns and private sector innovation in antiviral therapies contribute to consistent demand.
Canada Hepatitis B Infection Market Insight
The Canada hepatitis B infection market accounted for 25.6% of the North America market revenue in 2024. The country benefits from a publicly funded healthcare system, universal vaccination programs for children, and a strong emphasis on immigrant screening and maternal testing. Ongoing collaborations between government and research institutions have improved access to diagnostics and treatment for chronic HBV, particularly in underserved and Indigenous communities.
Mexico Hepatitis B Infection Market Insight
The Mexico hepatitis B infection market contributed 16.5% of the regional market share in 2024 and is expected to experience the fastest CAGR of 6.9% during the forecast period. Growth is supported by increasing government investment in infectious disease prevention, enhanced vaccination programs for infants and adolescents, and the expansion of diagnostic access in rural areas. Public-private partnerships and educational initiatives are also helping raise awareness about hepatitis B and reduce stigma, contributing to earlier detection and improved treatment outcomes.
North America Hepatitis B Infection Market Share
The North America hepatitis B infection industry is primarily led by well-established companies, including:
- Gilead Sciences, Inc. (U.S.)
- GSK plc (U.K.)
- Dynavax Technologies (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Arrowhead Pharmaceuticals Inc. (U.S.)
- Arbutus Biopharma (Canada)
- Teva Pharmaceuticals, Inc. (Israel)
- Zydus Pharmaceuticals (India)
- Aurobindo Pharma (India)
- Lupin Pharmaceuticals, Inc. (India)
Latest Developments in North America Hepatitis B Infection Market
- In September 2024, Gilead Sciences and Genesis Therapeutics announced a strategic collaboration to discover and develop novel small molecule therapies using Genesis’ GEMS AI platform. Gilead gained exclusive rights to develop and commercialize products from this partnership
- In July 2024, Gilead Sciences, Inc. presented research data, showcasing long-term efficacy and safety of Biktarvy in diverse HIV populations, including Hispanic/Latine individuals and older adults with comorbidities. Investigational once-daily and weekly dosing regimens were also highlighted
- In February 2024, GSK completed its acquisition of Aiolos Bio, including the promising AIO-001 monoclonal antibody for severe asthma. GSK paid USD 1000 million upfront and up to USD 400 million in milestone payments, expanding its respiratory biologics portfolio
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA HEPATITIS B INFECTION MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 THERAPEUTICS LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTAL ANALYSIS
4.2 PORTER FIVE FORCES
5 NORTH AMERICA HEPATITIS B INFECTION MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN THE ASIA-PACIFIC REGION
5.2 NORTH AMERICA REGULATORY SCENARIO
5.3 EUROPE REGULATORY SCENARIO
5.4 MIDDLE EAST AND AFRICA REGULATORY SCENARIO
5.5 SOUTH AMERICA REGULATORY SCENARIO
6 PIPELINE ANALYSIS
7 EPIDEMILIOGY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASING PREVALENCE OF HEPATITIS B INFECTIONS
8.1.2 TECHNOLOGICAL ADVANCEMENTS IN DIAGNOSTICS
8.1.3 DEVELOPMENT OF COMBINATION THERAPIES FOR HEPATITIS B
8.1.4 STRATEGIC INITIATIVES BY COMPANIES FOR HEPATITIS B INFECTION
8.2 RESTRAINTS
8.2.1 SIDE EFFECTS AND DRUG RESISTANCE
8.2.2 INSUFFICIENT VACCINE COVERAGE FOR HEPATITIS B INFECTION
8.3 OPPORTUNITY
8.3.1 RISING NEW DRUG RELEASES AND INCREASING NEW DRUG PERMITS FOR HEPATITIS B
8.3.2 GOVERNMENT PROGRAMS TO RAISE AWARENESS OF HEPATITIS B INFECTION
8.3.3 ADVANCED RESEARCH AND DEVELOPMENT FOR CLINICAL TRIALS
8.4 CHALLENGES
8.4.1 THE COST OF HEPATITIS B TREATMENTS IS HIGH
8.4.2 STRINGENT REGULATORY POLICIES AND REGIONAL DISPARITIES IN TREATMENT ACCESS
9 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE
9.1 OVERVIEW
9.2 CHRONIC
9.3 ACUTE
10 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 VACCINE
10.2.1 HOSPITAL PHARMACIES
10.2.2 DRUGS STORES AND RETAIL PHARMACIES
10.2.3 ONLINE PHARMACIES
10.3 ANTIVIRAL DRUGS
10.3.1 TENOFOVIR ALAFENAMIDE FUMARATE (TAF)
10.3.2 TENOFOVIR DISOPROXIL FUMARATE (TDF)
10.3.3 ENTECAVIR
10.3.4 OTHERS
10.4 IMMUNE MODULATOR DRUGS
10.4.1 PEGYLATED INTERFERON
10.4.2 INTERFERON ALPHA
10.5 SURGERY
11 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY REGION
11.1 NORTH AMERICA
11.1.1 U.S
11.1.2 CANADA
11.1.3 MEXICO
12 NORTH AMERICA HEPATITIS B TREATMENT MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 GILEAD SCIENCES, INC.
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENT
14.2 GLAXOSMITHKLINE PLC
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENT
14.3 DYNAVAX TECHNOLOGIES CORPORATION
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 PRODUCT PORTFOLIO
14.3.5 RECENT DEVELOPMENTS
14.4 F. HOFFMAN-LA ROCHE LTD.
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENTS
14.5 BRISTOL-MYERS SQUIBB COMPANY
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENTS
14.6 ARROWHEAD PHARMACEUTICALS, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENTS
14.7 ARBUTUS BIOPHARMA
14.7.1 COMPANY SNAPSHOT
14.7.2 REVENUE ANALYSIS
14.7.3 PRODUCT PORTFOLIO
14.7.4 RECENT UPDATES
14.8 AUROBINDO PHARMA
14.8.1 COMPANY SNAPSHOT
14.8.2 REVENUE ANALYSIS
14.8.3 PRODUCT PORTFOLIO
14.8.4 RECENT UPDATES
14.9 LUPIN PHARMACEUTICALS, INC.
14.9.1 COMPANY SNAPSHOT
14.9.2 PRODUCT PORTFOLIO
14.9.3 RECENT UPDATES
14.1 MERCK & CO., INC.,
14.10.1 COMPANY SNAPSHOT
14.10.2 REVENUE ANALYSIS
14.10.3 PRODUCT PORTFOLIO
14.10.4 RECENT DEVELOPMENTS
14.11 NOVARTIS AG
14.11.1 COMPANY SNAPSHOT
14.11.2 REVENUE
14.11.3 PRODUCT PORTFOLIO
14.11.4 RECENT DEVELOPMENT
14.12 TEVA PHARMACEUTICAL INDUSTRIES
14.12.1 COMPANY SNAPSHOT
14.12.2 PRODUCT PORTFOLIO
14.12.3 RECENT DEVELOPMENTS
14.13 ZYDUS PHARMACEUTICALS, INC.
14.13.1 COMPANY SNAPSHOT
14.13.2 PRODUCT PORTFOLIO
14.13.3 REVENUE
14.13.4 RECENT DEVELOPMENT
15 QUESTIONNAIRE
16 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA CLINICAL TRIAL AND PIPELINE A-LYSIS AS PER THE COMPANY
TABLE 2 DISTRIBUTION OF PRODUCTS OR PROJECTS BY PHASE
TABLE 3 COUNTRY WISE EPIDEMIOLOGY FOR HEPATITIS B
TABLE 4 COST OF HEPATITIS B MEDICATIONS: BRAND VS. GENERIC PRICES
TABLE 5 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 6 NORTH AMERICA CHRONIC IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 7 NORTH AMERICA ACUTE IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 8 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 9 NORTH AMERICA VACCINE IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 10 NORTH AMERICA VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 11 NORTH AMERICA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 12 NORTH AMERICA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 13 NORTH AMERICA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 14 NORTH AMERICA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 15 NORTH AMERICA SURGERY IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 16 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY COUNTRY, 2022-2031 (USD MILLION)
TABLE 17 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 18 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 19 NORTH AMERICA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 20 NORTH AMERICA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 21 NORTH AMERICA VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 22 U.S. HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 23 U.S. HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 24 U.S. ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 25 U.S. IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 26 U.S. VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 27 CANADA HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 28 CANADA HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 29 CANADA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 30 CANADA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 31 CANADA VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 32 MEXICO HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 33 MEXICO HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 34 MEXICO ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 35 MEXICO IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 36 MEXICO VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA HEPATITIS B INFECTION MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA HEPATITIS B INFECTION MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA HEPATITIS B INFECTION MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA HEPATITIS B INFECTION MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA HEPATITIS B INFECTION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA HEPATITIS B INFECTION MARKET: MULTIVARIATE MODELLING
FIGURE 7 NORTH AMERICA HEPATITIS B INFECTION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA HEPATITIS B INFECTION MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA HEPATITIS B INFECTION MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA HEPATITIS B INFECTION MARKET: SEGMENTATION
FIGURE 11 TWO SEGMENTS COMPRISE THE NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 NORTH AMERICA HEPATITIS B INFECTION MARKET
FIGURE 15 CHRONIC SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA HEPATITIS B INFECTION MARKET IN 2024 & 2031
FIGURE 16 DROC ANALYSIS
FIGURE 17 BURDEN OF HBV INFECTION IN THE GENERAL POPULATION BY WHO REGION, 2019
FIGURE 18 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, 2023
FIGURE 19 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, 2024-2031 (USD MILLION)
FIGURE 20 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TREATMENT, 2023
FIGURE 23 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TREATMENT, 2024-2031 (USD MILLION)
FIGURE 24 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 NORTH AMERICA HEPATITIS B INFECTION MARKET BY TREATMENT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA HEPATITIS B INFECTION MARKET, SNAPSHOT
FIGURE 27 NORTH AMERICA HEPATITIS B TREATMENT MARKET: COMPANY SHARE 2023 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.