North America Medical Device Testing Market Analysis and Insights
Medical devices are crucial since they directly impact human lives. Medical device manufacturers follow testing, verification, and validation best practices to ensure the quality and reliability of medical equipment to provide safe and effective healthcare services to patients. There is a high-level overview of implementing medical device testing strategies. Multiple regulatory bodies and compliances govern medical devices. On the other hand, end users expect exceptional performance, effectiveness, and safety from the device that they use. This compels medical device manufacturers to create and deploy testing strategies that work across the development cycle - from concept and design to production.
The rising need for the verification and validation of medical devices globally has enhanced the growth of the market. Some of the major market players are highly focusing on various service launches and service approvals during this crucial period. In addition, the increasing demand for in-vitro tests is also contributing to the rising demand for medical device testing. The North America medical device testing market is growing in the forecast year due to a rise in market players and the availability of advanced services.
However, the barriers to the local development of medical devices and the high cost of medical devices might hamper the growth of the North America medical device testing market in the forecast period.
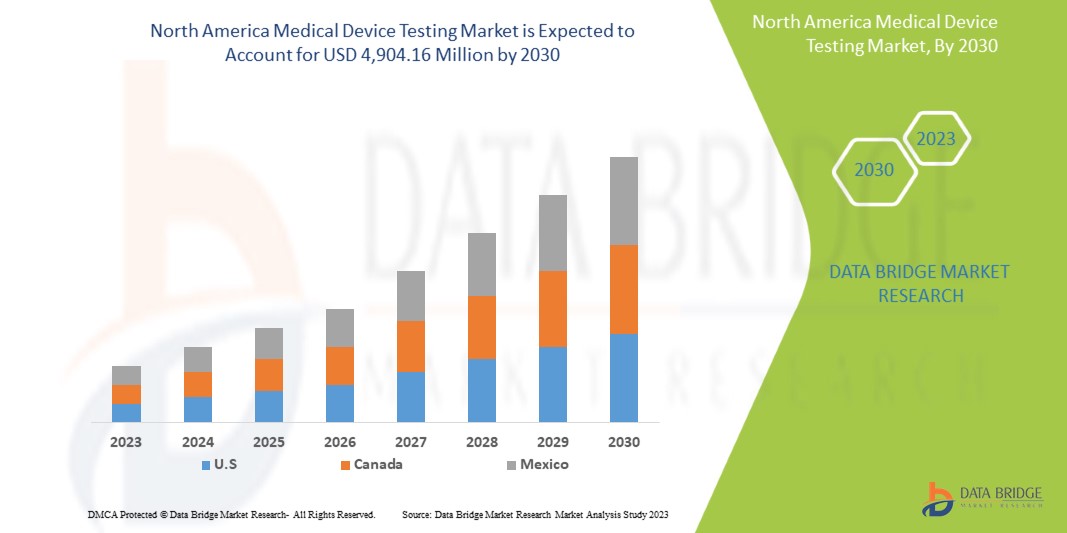
Data Bridge Market Research analyzes that the North America medical device testing market is expected to reach the value of USD 4,904.16 million by 2030, at a CAGR of 12.0% during the forecast period.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
Service Type (Testing Services, Inspection Service and Certification Services), Testing Type (Physical Testing, Chemical/Biological Testing, Cybersecurity Testing, Microbiology Testing and Sterility Testing, and Others), Phase (Preclinical and Clinical), Sourcing Type (In-House and Outsourced), Device Class (Class I, Class II and Class III), Product (Active Implant Medical Device, Active Medical Device, Non-active Medical Device, In vitro Diagnostics Medical Device, Opthalmic Medical Device, Orthopedic and Dental Medical Device, Vascular Medical Device, and Others) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Pace, Charles River Laboratories., Biomedical Device Labs, UL LLC, North American Science Associates, LLC, WuXi AppTec, NSF., Laboratory Corporation of America Holdings, Eurofins Scientific, NELSON LABORATORIES, LLC- A SOTERA HEALTH COMPANY, Gateway Analytical., Element Materials Technology, Hohenstein, Cigniti, Q Laboratories, and IMR Test Labs among others |
Market Definition
Medical device testing is the process of demonstrating that the device is reliably and safely performed in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity, chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This usually includes dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market, such as inspection services, certification services, and others.
North America Medical Device Testing Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Rising Need for Verification Validation of Medical Devices
Medical device testing is the process of demonstrating that the device will reliably and safely perform diagnosis and treatment. The demand for medical devices is constantly rising as there is a high prevalence of various diseases seen among various regions across the globe.
The methods of verification and validation are widespread and extensively being used in the healthcare industry. In general, verification is the developmental phase of a product if it complies with the specified requirements, whereas validation checks if the intended use has been met and, thus, usability specifics are fulfilled. The most common types of verification and validation for medical devices are design, process, and software verification and validation.
Medical devices are also becoming smaller and more complex in design using advanced, engineered plastics. This makes the process of validation and verification (V&V) all the more important. The results in better repeatability, fewer mistakes, less rework and redesign, faster time to market, improved competitiveness, and lower production costs. Additionally, increasing standards and regulations about medical device validation and verification services are anticipated to drive the growth of the medical device testing market.
- Increasing Demand for In-Vitro Tests
In vitro diagnostics (IVD) are tests done on samples such as blood or tissue that have been taken from the human body. In vitro diagnostics can detect diseases or other conditions and can be used to monitor a person’s overall health to help cure, treat, or prevent diseases.
In-vitro tests are used in various disease detections, such as HIV infections, malaria, and hepatitis, among others. The prevalence of such diseases is rapidly increasing across the globe, which leads to the increasing demand for in-vitro tests and various medical devices. As medical devices have become widely available for various diseases due to the rapid manufacturing of medical testing companies, it is a major factor driving the growth of the market.
Opportunities
- Rise in Healthcare Expenditure
Healthcare expenditure has increased worldwide as disposable income in various countries is increasing. Moreover, to accomplish the population requirements, government bodies and healthcare organizations are taking the initiative to accelerate healthcare expenditure. The rise in healthcare expenditure simultaneously helps healthcare settings to improve their treatment facilities for various disorders in the countries.
Growing healthcare expenditure is also beneficial for further economic growth and healthcare sector growth, and it is primarily fruitful as it significantly affects the development of better and more advanced medical products. Therefore, the surge in healthcare expenditure is a greater opportunity for the North America medical device testing market growth.
- Development in Ai And Iot in Various Medical Devices
Artificial intelligence (AI) is a powerful and ever-emerging technology that has the potential to improve capabilities across a multitude of industries. Medical devices with artificial intelligence hold the promise of revolutionizing the healthcare industry, helping medical professionals more accurately and effectively diagnose and treat their patients and improve their overall care. Along with its benefits, medical device artificial intelligence also faces challenges, including the need for regulation to keep up with technological advancement.
Thus, the North America medical device testing market is witnessing a series of developments in drug-device combination, personalized medicine, and elevated adaption of various portable and wearable medical devices. Implementing technological advancements such as IoT and Artificial Intelligence in various devices is a key growth parameter for the global medical device testing service market, creating a huge opportunity.
Challenges/ Restraints
- High Competition in Medical Technology Industry
Many global companies face significant competition from many companies, from large medical device manufacturing companies with multiple product lines. Several players have wide financial and marketing resources than others. The common competition in healthcare involves various elements such as price, quality, convenience, and brand name. Competition can also be based on new technologies and innovation. In general, competition reduces inefficiencies that would otherwise yield high costs of output, which are eventually passed to consumers by high costs of health care and delivery. Products on the market are marketed based on product specifications, product consistency, supply, and price. Consumers, however, benefit from competition that offers rewards for continuing product upgrades and facilitates a higher degree of service efficiency among industry participants. The company’s ability to compete in the market is also impacted by changes in consumer preferences and requirements, such as the growing demand for more eco-friendly medical devices that can be easily dumped without harming the environment and the products incorporated with digital capabilities. This shows high competition in the market, which is a major challenge for the market.
- Barriers to the Local Development of Medical Devices
While promoting standards and control of medical devices is essential, excessive regulations, particularly in the domestic context, can also act as a barrier to local innovation of these devices.
It can potentially hinder domestic innovation by subjecting new technologies to a lengthy and expensive licensing procedure, subsequently increasing the cost and the time that local manufacturers have to spend in addition to the manufacturing cost of the medical equipment. A few products, which are of significant value to low-income countries, may be removed from the market due to the perceived risks associated with their use.
Various factors, such as licensing procedures, the cost of the medical equipment, and various other regulations for the production of medical devices faced by local development manufacturers, all these causes may hamper the growth of the medical device testing market.
Recent Developments
- In April 2021, TÜV SÜD announced that it had presented itself at Medtec LIVE to exhibit its ability to be a one-stop shop for medical device testing. The company’s services cover testing in electrical and functional safety, cyber security and software, EMC, and biocompatibility. The experts from TÜV SÜD featured in the program of the online trade show and congress with various talks, a live hack, and an elevator pitch.
- In June 2020, Intertek announced the expansion of its personal protective equipment services to include precertification testing of N95 respirators to requirements set by the National Institute for Occupational Safety and Health (NIOSH). The new services are the result of the successful accreditation to NIOSH Standard Test Protocols, in accordance with ISO 17025. With these new services, Intertek expands upon its solutions and resources to support customers and the global community during the COVID-19 pandemic.
North America medical device testing market Scope
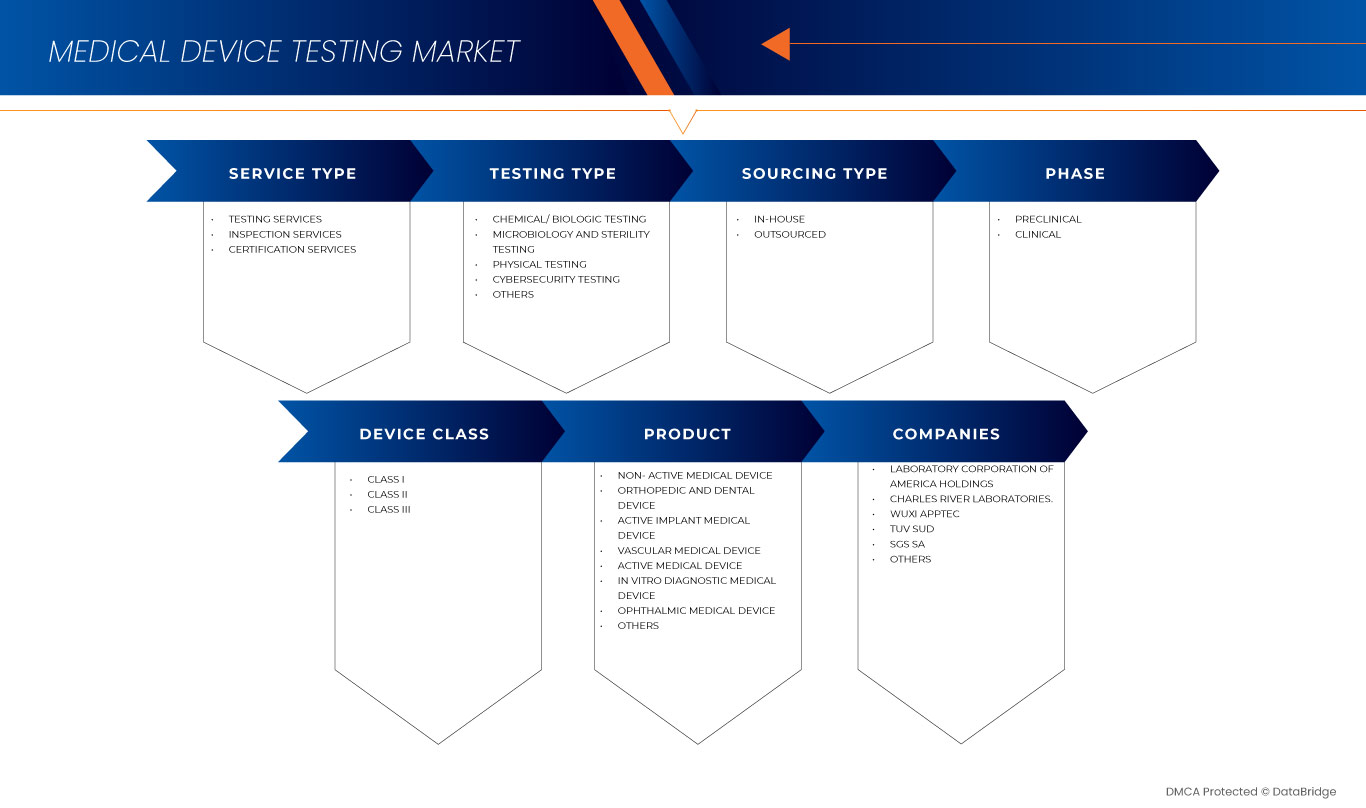
North America medical device testing market is segmented into service type, testing type, phase, sourcing type, device class, and product. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Service Type
- Testing Services
- Inspection Services
- Certification Services
On the basis of service type, the North America medical device testing market is segmented into testing services, inspection services, and certification services.
Testing Type
- Physical Testing
- Chemical/Biological Testing
- Cybersecurity Testing
- Microbiology And Sterility Testing
- Others
On the basis of testing type, the North America medical device testing market is segmented into physical testing, chemical/biological testing, cybersecurity testing, microbiology and sterility testing, and others.
Phase
- Preclinical
- Clinical
On the basis of phase, the North America medical device testing market is segmented into preclinical and clinical.
Sourcing Type
- In-House
- Outsourced
On the basis of sourcing type, the North America medical device testing market is segmented into in-house and outsourced
Device Class
- Class I
- Class II
- Class III
On the basis of device class, the North America medical device testing market is segmented into class I, class II, and class III.
Product
- Active Implant Medical Device
- Active Medical Device
- Non-Active Medical Device
- In Vitro Diagnostic Medical Device
- Ophthalmic Medical Device
- Orthopedic and Dental Medical Device
- Vascular Medical Device
- Others
On the basis of product, the North America medical device testing market is segmented into active implant medical device, active medical device, non-active medical device, in vitro diagnostics medical device, opthalmic medical device, orthopedic and dental medical device, vascular medical device, and others.
North America Medical Device Testing Market Regional Analysis/Insights
North America medical device testing market is segmented into service type, testing type, phase, sourcing type, device class, and product.
The countries covered in this market report are the U.S., Canada, and Mexico.
The U.S. is expected to dominate the market due to the high prevalence of medical device testing in the region and the rise in healthcare expenditure.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Medical Device Testing Market Share Analysis
North America medical device testing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the North America medical device testing market.
Some of the major players operating in the North America medical device testing market are Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Pace, Charles River Laboratories., Biomedical Device Labs, UL LLC, North American Science Associates, LLC, WuXi AppTec, NSF., Laboratory Corporation of America Holdings, Eurofins Scientific, NELSON LABORATORIES, LLC- A SOTERA HEALTH COMPANY, Gateway Analytical., Element Materials Technology, Hohenstein, Cigniti, Q Laboratories, and IMR Test Labs among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA MEDICAL DEVICE TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING NEED FOR VERIFICATION VALIDATION OF MEDICAL DEVICES
6.1.2 INCREASING DEMAND FOR IN-VITRO TESTS
6.1.3 ESCALATION IN INNOVATION AND TECHNOLOGIES
6.2 RESTRAINTS
6.2.1 BARRIERS TO THE LOCAL DEVELOPMENT OF MEDICAL DEVICES
6.2.2 HIGH COST OF MEDICAL DEVICES
6.3 OPPORTUNITIES
6.3.1 RISE IN HEALTHCARE EXPENDITURE
6.3.2 DEVELOPMENT IN AI AND IOT IN VARIOUS MEDICAL DEVICES
6.3.3 STRATEGIC INITIATIVES OF KEY PLAYERS
6.4 CHALLENGES
6.4.1 HIGH COMPETITION IN MEDICAL TECHNOLOGY INDUSTRY
6.4.2 LONG LEAD TIME FOR OVERSEAS QUALIFICATION
7 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE
7.1 OVERVIEW
7.2 TESTING SERVICES
7.3 INSPECTION SERVICES
7.4 CERTIFICATION SERVICES
8 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE
8.1 OVERVIEW
8.2 CHEMICAL/BIOLOGICAL TESTING
8.3 MICROBIOLOGY AND STERILITY TESTING
8.3.1 STERILITY TEST & VALIDATION
8.3.2 BIO BURDEN DETERMINATION
8.3.3 ANTIMICROBIAL ACTIVITY TESTING
8.3.4 PYROGEN & ENDOTOXIN TESTING
8.3.5 OTHERS
8.4 PHYSICAL TESTING
8.4.1 ELECTRICAL SAFETY TESTING
8.4.2 FUNCTIONAL SAFETY TESTING
8.4.3 EMC TESTING
8.4.4 ENVIRONMENTAL TESTING
8.4.5 OTHERS
8.5 CYBERSECURITY TESTING
8.6 OTHERS
9 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY PHASE
9.1 OVERVIEW
9.2 PRECLINICAL
9.3 CLINICAL
10 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE
10.1 OVERVIEW
10.2 OUTSOURCED
10.3 IN-HOUSE
11 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS
11.1 OVERVIEW
11.2 CLASS I
11.3 CLASS III
11.4 CLASS II
12 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 NON-ACTIVE MEDICAL DEVICE
12.3 ORTHOPEDIC AND DENTAL MEDICAL DEVICE
12.4 ACTIVE IMPLANT MEDICAL DEVICE
12.5 VASCULAR MEDICAL DEVICE
12.6 ACTIVE MEDICAL DEVICE
12.7 IN-VITRO DIAGNOSTICS MEDICAL DEVICE
12.8 OPTHALMIC MEDICAL DEVICE
12.9 OTHERS
13 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY GEOGRAPHY
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 LABORATORY CORPORATION OF AMERICA HOLDINGS
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 CHARLES RIVER LABORATORIES.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 TUV SUD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 WUXI APPTEC
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 SGS SA
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 NORTH AMERICAN SCIENCE ASSOCIATES, LLC
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 HOHENSTEIN
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 BIOMEDICAL DEVICE LABS
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BUREAU VERITAS
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 CIGNITI
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENTS
16.11 ELEMENT MATERIALS TECHNOLOGY
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 EUROFINS SCIENTIFIC
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 GATEWAY ANALYTICAL.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 IMR TEST LABS
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 INTERTEK GROUP PLC
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 NELSON LABORATORIES, LLC- A SOTERA HEALTH COMPANY
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 NSF.
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 PACE
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 Q LABORATORIES
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 TUV RHEINLAND
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENTS
16.21 UL LLC
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 FDA REGULATIONS BASED ON DEVICES TYPE
TABLE 2 PRICES OF ESSENTIAL MEDICAL DEVICES
TABLE 3 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA MEDICAL DEVICE TESTING MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 12 U.S. MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2021-2030 (USD MILLION)
TABLE 13 U.S. MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 14 U.S. MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 15 U.S. PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 16 U.S. MEDICAL DEVICE TESTING MARKET, BY PHASE, 2021-2030 (USD MILLION)
TABLE 17 U.S. MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2021-2030 (USD MILLION)
TABLE 18 U.S. MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2021-2030 (USD MILLION)
TABLE 19 U.S. MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 20 CANADA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2021-2030 (USD MILLION)
TABLE 21 CANADA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 22 CANADA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 23 CANADA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 24 CANADA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2021-2030 (USD MILLION)
TABLE 25 CANADA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2021-2030 (USD MILLION)
TABLE 26 CANADA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2021-2030 (USD MILLION)
TABLE 27 CANADA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 MEXICO MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2021-2030 (USD MILLION)
TABLE 29 MEXICO MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 30 MEXICO MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 31 MEXICO PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2021-2030 (USD MILLION)
TABLE 32 MEXICO MEDICAL DEVICE TESTING MARKET, BY PHASE, 2021-2030 (USD MILLION)
TABLE 33 MEXICO MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2021-2030 (USD MILLION)
TABLE 34 MEXICO MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2021-2030 (USD MILLION)
TABLE 35 MEXICO MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: SEGMENTATION
FIGURE 11 INCREASING DEMAND FOR IN-VITRO TESTS AND DEVELOPMENT IN AI AND IOT IN VARIOUS MEDICAL DEVICES ARE EXPECTED TO DRIVE THE NORTH AMERICA MEDICAL DEVICE TESTING MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 TESTING SERVICES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA MEDICAL DEVICE TESTING MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF GLOBAL MEDICAL DEVICE TESTING MARKET
FIGURE 14 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, 2022
FIGURE 15 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, 2022
FIGURE 19 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PHASE, 2022
FIGURE 23 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PHASE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PHASE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PHASE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, 2022
FIGURE 27 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, 2022
FIGURE 31 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, LIFELINE CURVE
FIGURE 34 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PRODUCT, 2022
FIGURE 35 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 38 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: SNAPSHOT (2022)
FIGURE 39 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2022)
FIGURE 40 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2023 & 2030)
FIGURE 42 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE (2023-2030)
FIGURE 43 NORTH AMERICA MEDICAL DEVICE TESTING MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.