North America Multiple Sclerosis Treatment Market
Market Size in USD million
CAGR :
% 
 USD
2,879.60 million
USD
5,737.78 million
2022
2030
USD
2,879.60 million
USD
5,737.78 million
2022
2030
| 2023 –2030 | |
| USD 2,879.60 million | |
| USD 5,737.78 million | |
|
|
|
North America Multiple Sclerosis Treatment Market Analysis and Size
The increasing burden of numerous infectious diseases on the global healthcare system mainly responsible for the market growth. Multiple sclerosis treatment demand has increased as compared to the precise year with growing incidence of multiple sclerosis along with the rising need for better multiple sclerosis treatment options and growing government initiatives. Governments and non-government organizations in developed and developing countries are putting their efforts to increase awareness of multiple sclerosis and giving major funds for drug research.
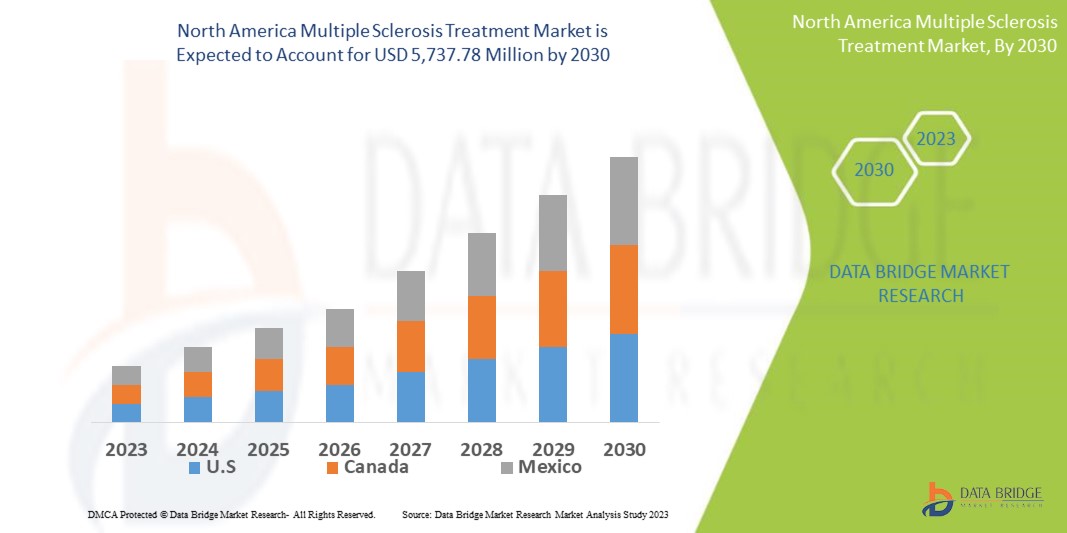
Data Bridge Market Research analyses that the multiple sclerosis treatment market is expected to reach USD 5,737.78 million by 2030, which is USD 2,879.6 million in 2022, and is expected to undergo a CAGR of 9% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
North America Multiple Sclerosis Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Disease Type (Relapsing–Remitting Multiple Sclerosis (RRMS), Secondary Progressive Multiple Sclerosis (SPMS), Primary Progressive Multiple Sclerosis (PPMS), Severe Relapsing–Remitting Multiple Sclerosis (RES)), Treatment (Preventive Therapies, Abortive Therapies/Treatment of Acute Exacerbations, Symptomatic Therapies), Drug Type (Branded, Generic), End User (Hospitals, Trauma Centres, Ambulatory Surgical Centres and Other) |
|
Countries Covered |
U.S., Canada and Mexico in North America |
|
Market Players Covered |
AbbVie Inc. (U.S.), Bausch Health Companies Inc. (Canada), Biora Therapeutics, Inc (U.S.), Boehringer Ingelheim International Gmbh (Germany), Amgen Inc. (U.S.), Pfizer Inc (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Mylan N.V. (U.S.), Novartis AG (Switzerland), Bayer AG (Germany), Bristol Myers Squibb Company (U.S.), Biogen (U.S.), Teva Pharmaceutical Industries Ltd (Israel), Takeda Pharmaceutical Company Limited (Japan), Jazz Pharmaceuticals, Inc (U.K.), Abbott (U.S.), Bio-Rad Laboratories Inc. (U.S.), Mylan N.V. (U.S.) |
|
Market Opportunities |
|
Market Definition
Multiple sclerosis (MS) is a chronic and inflammatory immune-mediated condition that can cause axonal transection, demyelination, and neurodegeneration of the spinal cord and brain nerve cells. The symptoms depends on the amount of damaged nerves and the form and position of affected nerves. The multiple sclerosis causes numerous types of symptoms which includes pain, fatigue, vision loss, and impaired coordination. This disease duration, symptoms, and severity vary from person to person.
North America Multiple Sclerosis Treatment Market Dynamics
Drivers
- Increasing Drug Development Processes by Several Market Players
Several pharmaceutical companies have also been investing heavily in the drug development process. They are planning to target different indications to attract a large number of customers. For instance, the drugs developed for multiple sclerosis include Secondary progressive MS, Primary progressive MS, Relapsing-remitting MS, and Myelin repair or neuroprotection. For instance, Novartis received European Commission approval for Kesimpta (ofatumumab) in March 2021, for the treatment of relapsing forms of multiple sclerosis (RMS) in adults with active disease defined by clinical or imaging features. Thus, this factor boosts the market growth.
- Rising Demand of Branded Drugs
The rising demand of branded drugs increase the growth of the market. The demand is increasing because of the large number of products launched from time-to-time by numerous companies, making it difficult for generics to overpower it. Furthermore, the FDA approved the branded drugs when a New Drug Application is submitted with proof of stability, efficacy, dosage form, manufacture, safety, labelling, chemistry, and packaging, which largely increases its demand. Thus, it will boost the market growth.
Opportunities
- Increasing Product Launches
There have been increasing drug launches to treat multiple sclerosis, enhancing the market growth. For instance, Mylan N.V. announced the launch of its first FDA-approved generic drug dimethyl fumarate delayed-release capsules 120 mg and 240 mg used for the treatment of relapsing forms of multiple sclerosis (MS). The newly launched drug is almost similar therapeutically to the Biogen's Tecfidera capsules. This new FDA approved drug launched by the company during the COVID-19 pandemic has increased its demand in the market.
- Growing Demand of Oral Route of Administration
Several novel oral drugs recently approved for treating multiple sclerosis represent major advances in therapy. The oral route of administration offers patient satisfaction and increases therapeutic compliance. For instance, The European Commission (EC) approved Aubagio (teriflunomide) in June 2021, for the treatment of pediatric patients aged 10 to 17 years suffering from relapsing-remitting multiple sclerosis (RRMS). Aubagio is the first oral multiple sclerosis (MS) therapy applicable for first-line treatment of children and adults with MS in the European Union. Additionally, Bristol-Myers Squibb Company received U.S. FDA approval for ZEPOSIA (ozanimod) 0.92 mg in March 2020, for the treatment of adults with relapsing forms of multiple sclerosis (RMS), along with relapsing-remitting disease, clinically isolated syndrome, and active secondary progressive disease. Thus, this factor enhances the market growth rate.
Restraints/Challenges
- Side-Effects of Multiple Sclerosis Medications
There are various adverse effects that are associated with multiple sclerosis that impedes the growth of the market. Effects such as flu-like symptoms, chest pain, fluctuation in heart rate, rare brain infection, and chemotherapy-like effects hampers patients' adoption of multiple sclerosis drugs. This factor is anticipated to limit the market growth.
This multiple sclerosis treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the multiple sclerosis treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments:
- In 2020, Genzyme Corporation signed an acquisition agreement with Principia Biopharma Inc. The main motive behind this acquisition was to improve its research activities in the field of multiple sclerosis and other immune-mediated diseases.
- In 2020, Novartis announced that the U.S. FDA approval for Kesimpta in the form of an injection for subcutaneous use for treating the relapsing forms of multiple sclerosis (RMS), which includes clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
North America Multiple Sclerosis Treatment Market Scope
The multiple sclerosis treatment market is segmented on the basis of disease type, treatment, drug type, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Disease Type
- Relapsing–Remitting Multiple Sclerosis (RRMS)
- Secondary Progressive Multiple Sclerosis (SPMS)
- Primary Progressive Multiple Sclerosis (PPMS)
- Severe Relapsing–Remitting Multiple Sclerosis (RES)
Treatment
- Preventive Therapies
- Abortive Therapies/Treatment of Acute Exacerbations
- Symptomatic Therapies
Drug Type
- Branded
- Generic
Route of Administration
- Oral
- Parenteral
End-user
- Hospital and Clinics
- Diagnostic Laboratories
- Others
Multiple Sclerosis Treatment Regional Analysis/Insights
The multiple sclerosis treatment market is analyzed and market size insights and trends are provided by disease type, treatment, drug type and end-user as referenced above.
The countries covered in the multiple sclerosis treatment market report are U.S., Canada and Mexico in North America.
U.S. is expected to lead the market in the forecast period of 2023 to 2030 because of the introduction of latest treatment options for multiple sclerosis in the region. The increasing R&D for drug development in the region is also increasing the growth of the market. The increased awareness of people related with multiple sclerosis and its treatment is aiding in leading the market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Multiple Sclerosis Treatment Market Share Analysis
The multiple sclerosis treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to multiple sclerosis treatment market.
Some of the major players operating in the multiple sclerosis treatment market are:
- AbbVie Inc. (U.S.)
- Bausch Health Companies Inc. (Canada)
- Biora Therapeutics, Inc (U.S.)
- Boehringer Ingelheim International Gmbh (Germany)
- Amgen Inc. (U.S.)
- Pfizer Inc (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland)
- Bayer AG (Germany)
- Bristol Myers Squibb Company (U.S.)
- Biogen (U.S.)
- Teva Pharmaceutical Industries Ltd (Israel)
- Takeda Pharmaceutical Company Limited (Japan)
- Jazz Pharmaceuticals, Inc (U.K.)
- Abbott (U.S.)
- Bio-Rad Laboratories Inc. (U.S.)
- Mylan N.V. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.