North America Prophylaxis Of Organ Rejection Market
Market Size in USD Million
CAGR :
% 
 USD
1,092.43 Million
USD
1,560.26 Million
2021
2029
USD
1,092.43 Million
USD
1,560.26 Million
2021
2029
| 2022 –2029 | |
| USD 1,092.43 Million | |
| USD 1,560.26 Million | |
|
|
|
Market Analysis and Insights: North America Prophylaxis of Organ Rejection Market
North America prophylaxis of organ rejection market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 4.7% in the forecast period of 2022 to 2029 and is expected to reach USD 1,560.26 million by 2029 from USD 1,092.43 million in 2021. The rising prevalence of organ transplantation and increased use of immunosuppressant are likely to be the major drivers which propel the demand of the market in the forecast period.
The term prophylaxis means treatment given or action taken to prevent disease. The prophylaxis of organ rejection, refers to the prevention of organ rejection by use of medicines. The patients who undergo transplantation, must be maintained on an immunosuppression regimen for rejection prophylaxis to help ensure graft survival. The transplant rejection is a process, in which a transplant recipient’s immunity the current rejection prophylaxis implements use of calcineurin inhibitors, mTOR inhibitors, antimetabolite agents, and corticosteroids. The treatment agents improve the short term consequences of the organ transplantation, but certain improvements. The recipient patients, who have undergone solid organ transplantation, should take anti-rejection medicines. This is because the immunity system would destroy the transplanted organ.
The driving factors responsible for the growth of the North America prophylaxis of organ rejection market are the increased prevalence of organ transplantation, rise in surgical procedures, rise in recipient population and product launches. However, the factors that are expected to restrain the market are the rise in the cost of the transplantation procedure, lack of awareness about organ transplantation and the risks included while the patient receives the transplanted organ.
- On the other hand, strategic initiatives by market players, rise in research and developments and use of immunosuppressant may act as an opportunity for the growth of the North America prophylaxis of organ rejection market. The need for skilled expertise and the regulatory approval may create challenges for the North America prophylaxis of organ rejection market. There are recent developments related to the North America prophylaxis of organ rejection market.
The North America prophylaxis of organ rejection market report provides details of market share, new developments, and impact of domestic and localised market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
North America Prophylaxis of Organ Rejection Market Scope and Market Size
North America prophylaxis of organ rejection market is categorized into seven segments cause, treatment, route of administration, organ, patient type, end user and distribution channel.
The growth among segments helps you analyse niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of cause, the North America prophylaxis of organ rejection market is segmented into epidemiologic exposure, antibacterial prophylaxis, prophylaxis against other pathogens and others. In 2022, the epidemiologic exposure segment is expected to dominate the North America prophylaxis of organ rejection market due to availability of direct information and presence of pathogens related to prophylaxis of organ rejection in the U.S.
- On the basis of treatment, the North America prophylaxis of organ rejection market is segmented into outpatient immunosuppressant, inpatient immunosuppressant and others. In 2022, the outpatient immunosuppressant segment is expected to dominate the North America prophylaxis of organ rejection market, since it can achieve the sustained and specific immune response against damaged cells and availability of cyclosporine in the U.S. and Canada.
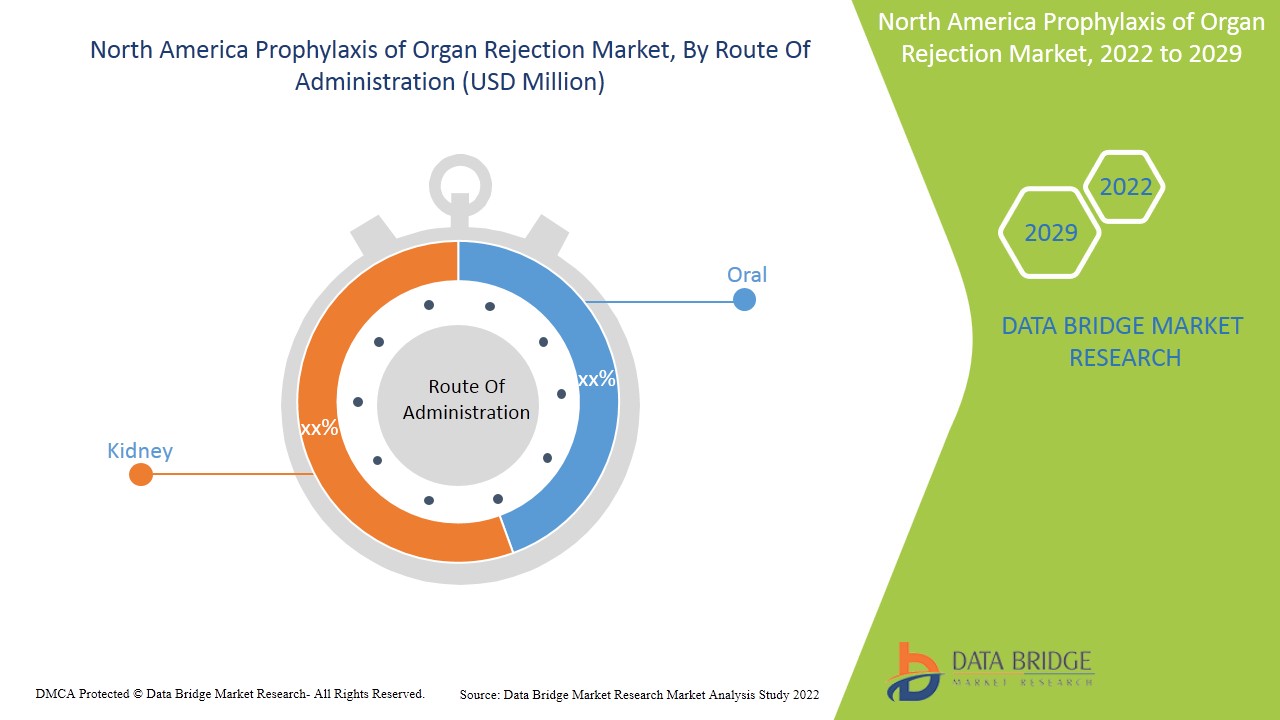
- On the basis of route of administration, the North America prophylaxis of organ rejection market is segmented into oral and intravenous. In 2022, the oral segment is expected to dominate the North America prophylaxis of organ rejection due to increased availability and intake of oral tablets and capsules.
- On the basis of organ, the North America prophylaxis of organ rejection market is segmented into kidney, liver, heart, lung and others. In 2022, the kidney segment is expected to dominate the North America prophylaxis of organ rejection due to increased incidence of kidney failure and presence of reimbursement policies such as Medicare, for the recipient’s insurance.
- On the basis of patient type, the North America prophylaxis of organ rejection market is segmented into pediatric and adults. In 2022, the adults segment is expected to dominate the North America prophylaxis of organ rejection market, due to increased prevalence of chronic diseases and genetic conditions of the adult patients, weak immune system in adults and waiting list of adult recipients more than the waiting list of children and women in New Mexico and the U.S.
- On the basis of end user, the North America prophylaxis of organ rejection market is segmented into hospitals, clinics, home healthcare and others. In 2022, the hospitals segment is expected to dominate the North America prophylaxis of organ rejection market due to rise in medical care and collaboration with other hospitals in North America for delivery of the donor organs to the recipient patients are predicted to dominate the market.
- On the basis of distribution channel, the North America prophylaxis of organ rejection market is segmented into direct tenders, pharmacy stores and others. In 2022, the direct tenders segment is expected to dominate the North America prophylaxis of organ rejection market due to surge in demand of outpatient immunosuppressant by pharmaceutical companies and guaranteed payment, are predicted to dominate the market.
North America Prophylaxis of Organ Rejection Market Country Level Analysis
North America prophylaxis of organ rejection market is categorized into seven segments cause, treatment, route of administration, organ, patient type, end user and distribution channel.
The countries covered in the North America prophylaxis of organ rejection market report are the U.S., Canada and Mexico.
- In 2021, U.S. is dominating due to the presence of the largest consumer market with high GDP. Moreover, the U.S. has the highest household spending in the world and offers trade agreements with several countries making it the largest market for consumer products including products for prophylaxis of organ rejection due to the presence of major market players and increased technological advancement in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Growth Potential for Prophylaxis of Organ Rejection In Emerging Economies and the Strategic Initiatives by Market Players are Creating New Opportunities in the North America Prophylaxis of Organ Rejection market
North America prophylaxis of organ rejection market also provides you with detailed market analysis for every country growth in particular industry with the Prophylaxis of Organ Rejection sales. The impact of advancement in the Prophylaxis of Organ Rejection and changes in regulatory scenarios with their support for the Prophylaxis of Organ Rejection market. The data is available for historic period 2020 to 2021.
Competitive Landscape and North America Prophylaxis of Organ Rejection Market Share Analysis
North America prophylaxis of organ rejection market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to the North America Prophylaxis of Organ Rejection market.
The major companies providing the prophylaxis of organ rejection are Ossium Health, Inc, Altavant Sciences, Inc, Concord Biotech, WOCKHARDT, SEBELA PHARMACEUTICALS, Veloxis Pharmaceuticals, Inc, Astellas Pharma Inc, Novartis AG, Bristol-Myers Squibb Company, Dr Reddy’s Laboratories, Ltd, Viatris Inc, Strides Pharma Science Limited, Glenmark, Biocon, Pfizer Inc, Mayne Pharma Group Limited, Hikma Pharmaceuticals PLC, AbbVie Inc, Apotex Inc, Teva Pharmaceutical U.S.A Inc, Amneal Pharmaceuticals LLC, Zydus Pharmaceuticals, Inc., Novitium Pharma, ZHEJIANG HISUN PHARMACEUTICAL Co., LTD., CSL Behring among others.
DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
The strategic initiatives by market players along with new technological advancements for North America prophylaxis of organ rejection is bridging the gap for use of medicines to avoid organ rejection.
For instance,
- In January 2020, Altavant Sciences, Inc, had acquired Onspira Therapeutics. Altavant Sciences Inc had developed OSP-101 for the treatment of bronchiolitis obliterans syndrome, the leading cause of morbidity and mortality in post-lung transplant patients. The acquisition would expand the product portfolio and to include OSP-101, for post market approval.
Collaboration, joint ventures and other strategies by the market player is enhancing the company market in the prophylaxis of organ rejection market which also provides the benefit for organisation to improve their offering for North America prophylaxis of organ rejection market.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 BY CAUSE SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: EPIDEMIOLOGY SNAPSHOT
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN PREVALENCE OF ORGAN TRANSPLANTATIONS
5.1.2 RISE IN CHRONIC DISEASES
5.1.3 RISE IN CLINICAL TRIALS
5.1.4 RISE IN RECIPIENT PATIENTS
5.1.5 PRODUCT APPROVALS
5.2 RESTRAINTS
5.2.1 RISE IN COST OF TRANSPLANTATION PROCEDURE
5.2.2 LACK OF AWARENESS ABOUT ORGAN TRANSPLANTATION
5.2.3 RISKS INCLUDED WITH THE PATIENT RECEIVES TRANSPLANTED ORGAN
5.2.4 ETHICAL ISSUES RELATED TO ORGAN TRANSPLANTATION
5.3 OPPORTUNITIES
5.3.1 RISE IN RESEARCH AND DEVELOPMENTS
5.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
5.3.3 RISE IN USE OF IMMUNOSUPPRESSANT
5.4 CHALLENGES
5.4.1 SHORTAGE OF SKILLED PROFESSIONALS REQUIRED FOR THE USE OF PROPHYLAXIS OF ORGAN REJECTION
5.4.2 STRINGENT REGULATIONS
6 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE
6.1 OVERVIEW
6.2 EPIDEMIOLOGIC EXPOSURE
6.2.1 HOSPITAL EXPOSURE
6.2.2 COMMUNITY EXPOSURE
6.3 ANTIBACTERIAL PROPHYLAXIS
6.3.1 POST-TRANSPLANT PROPHYLAXIS
6.3.2 PERIOPERATIVE ANTIBACTERIAL PROPHYLAXIS
6.4 PROPHYLAXIS AGAINST OTHER PATHOGENS
6.5 OTHERS
7 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT
7.1 OVERVIEW
7.2 OUTPATIENT IMMUNOSUPPRESSANT
7.2.1 CALCINEURIN INHIBITORS
7.2.1.1 CYCLOSPORINE
7.2.1.2 TACROLIMUS
7.2.1.2.1 IMMEDIATE RELEASE
7.2.1.2.2 EXTENDED RELEASE
7.2.2 CORTICOSTEROIDS
7.2.2.1 METHYLPREDNISOLONE
7.2.2.2 PREDNISONE
7.2.2.3 OTHERS
7.2.3 MYCOPHENOLIC ACIDS
7.2.3.1 MYCOPHENOLATE MOFETIL
7.2.3.2 MYCOPHENOLATE SODIUM
7.2.4 MTOR INHIBITORS
7.2.4.1 SIROLIMUS
7.2.4.2 EVEROLIMUS
7.2.5 AZATHIOPRINE
7.2.6 OTHERS
7.3 INPATIENT IMMUNOSUPPRESSANT
7.3.1 BELATACEPT
7.3.2 BASILIXIMAB
7.3.3 OTHERS
7.4 OTHERS
7.4.1 ERYTHROPOIESIS-STIMULATING AGENTS
7.4.1.1 ERYTHROPOIETIN
7.4.1.2 DARBEPOETIN
7.4.2 ANTI-VIRAL AGENTS
7.4.2.1 VALGANCICLOVIR
7.4.2.2 LAMIVUDINE
7.4.2.3 ADEFOVIR
7.4.2.4 OTHERS
7.4.3 OTHERS
8 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION
8.1 OVERVIEW
8.2 ORAL
8.2.1 TABLET
8.2.1.1 IMMEDIATE RELEASE
8.2.1.2 EXTENDED-RELEASE
8.2.2 ORAL SOLUTION
8.2.3 OTHERS
8.3 INTRAVENOUS
9 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ORGAN
9.1 OVERVIEW
9.2 KIDNEY
9.3 LIVER
9.4 HEART
9.5 LUNG
9.6 OTHERS
10 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 ADULTS
10.3 PEDIATRIC
11 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 HOME HEALTHCARE
11.5 OTHERS
12 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDERS
12.3 PHARMACY STORES
12.3.1 HOSPITAL PHARMACY
12.3.2 RETAIL PHARMACY
12.3.3 ONLINE PHARMACY
12.4 OTHERS
13 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 QUESTIONNAIRE
16 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA PROPHYLAXIS AGAINST OTHER PATHOGENS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA ORAL IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA ORAL IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA TABLET IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA INTRAVENOUS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA KIDNEY IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA LIVER IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA HEART IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA LUNG IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA ADULTS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA PEDIATRIC IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA HOSPITALS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA CLINICS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA HOME HEALTHCARE IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA DIRECT TENDERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 56 NORTH AMERICA CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 57 NORTH AMERICA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 58 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 59 NORTH AMERICA ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 60 NORTH AMERICA ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 61 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 62 NORTH AMERICA ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 63 NORTH AMERICA TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 64 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 65 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 66 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 67 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 68 NORTH AMERICA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 69 U.S. PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 70 U.S. EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 71 U.S. ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 72 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 73 U.S. OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 74 U.S. CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 75 U.S. TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 76 U.S. MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 77 U.S. MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 78 U.S. CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 79 U.S. INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 80 U.S. OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 81 U.S. ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 82 U.S. ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 83 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 84 U.S. ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 85 U.S. TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 86 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 87 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 88 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 89 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 90 U.S. PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 91 CANADA PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 92 CANADA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 93 CANADA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 94 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 95 CANADA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 96 CANADA CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 97 CANADA TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 98 CANADA MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 99 CANADA MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 100 CANADA CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 101 CANADA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 102 CANADA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 103 CANADA ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 104 CANADA ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 105 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 106 CANADA ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 107 CANADA TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 108 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 109 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 110 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 111 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 112 CANADA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 113 MEXICO PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 114 MEXICO EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 115 MEXICO ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 116 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 117 MEXICO OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 118 MEXICO CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 119 MEXICO TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 120 MEXICO MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 121 MEXICO MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 122 MEXICO CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 123 MEXICO INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 124 MEXICO OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 125 MEXICO ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 126 MEXICO ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 127 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 128 MEXICO ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 129 MEXICO TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 130 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 131 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 132 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 133 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 134 MEXICO PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: DBMR POSITION GRID
FIGURE 8 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED PREVALENCE OF ORGAN TRANSPLANTATION, RISE IN RECIPIENT PATIENTS, AND PRODUCT APPPROVALS ARE EXPECTED TO DRIVE NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET FROM 2022 TO 2029
FIGURE 13 EPIDEMIOLOGIC EXPOSURE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET FROM 2022 & 2029
FIGURE 14 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR PROPHYLAXIS OF ORGAN REJECTION MANUFACTURERS IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 15 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: INCIDENCE, 2021
FIGURE 16 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: INCIDENCE REGIONAL LEVEL, 2021
FIGURE 17 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: PREVALENCE (2018-2021) AT REGIONAL LEVEL SNAPSHOT
FIGURE 18 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: SURVIVAL OF PATIENT SNAPSHOT
FIGURE 19 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET
FIGURE 20 INCREASE COUNT OF KIDNEY TRANSPLANTATIONS IN THE U.S., 2020
FIGURE 21 TOTAL NUMBER OF ORGAN TRANSPLANTATION IN THE NORTH AMERICA SCENARIO, 2020
FIGURE 22 TOTAL NUMBER OF PATIENTS ON WAITING LIST VS TRANSPLANTS PERFORMED BY ORGAN IN 2020
FIGURE 23 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, 2021
FIGURE 24 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, 2020-2029 (USD MILLION)
FIGURE 25 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, CAGR (2022-2029)
FIGURE 26 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 27 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, 2021
FIGURE 28 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, 2020-2029 (USD MILLION)
FIGURE 29 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, CAGR (2022-2029)
FIGURE 30 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 31 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 32 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
FIGURE 33 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 34 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 35 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, 2021
FIGURE 36 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, 2020-2029 (USD MILLION)
FIGURE 37 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, CAGR (2022-2029)
FIGURE 38 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, LIFELINE CURVE
FIGURE 39 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, 2021
FIGURE 40 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, 2020-2029 (USD MILLION)
FIGURE 41 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 42 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 43 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, 2021
FIGURE 44 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 45 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, CAGR (2022-2029)
FIGURE 46 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, LIFELINE CURVE
FIGURE 47 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 48 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 49 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 50 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 51 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: SNAPSHOT (2021)
FIGURE 52 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY COUNTRY (2021)
FIGURE 53 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY COUNTRY (2022 & 2029)
FIGURE 54 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY COUNTRY (2021 & 2029)
FIGURE 55 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY CAUSE (2022-2029)
FIGURE 56 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.