North America Rx Dermatology Topical Drug Delivery Market
Market Size in USD Billion
CAGR :
% 
 USD
5.42 Billion
USD
9.86 Billion
2024
2032
USD
5.42 Billion
USD
9.86 Billion
2024
2032
| 2025 –2032 | |
| USD 5.42 Billion | |
| USD 9.86 Billion | |
|
|
|
|
Rx Dermatology Topical Drug Delivery Market Size
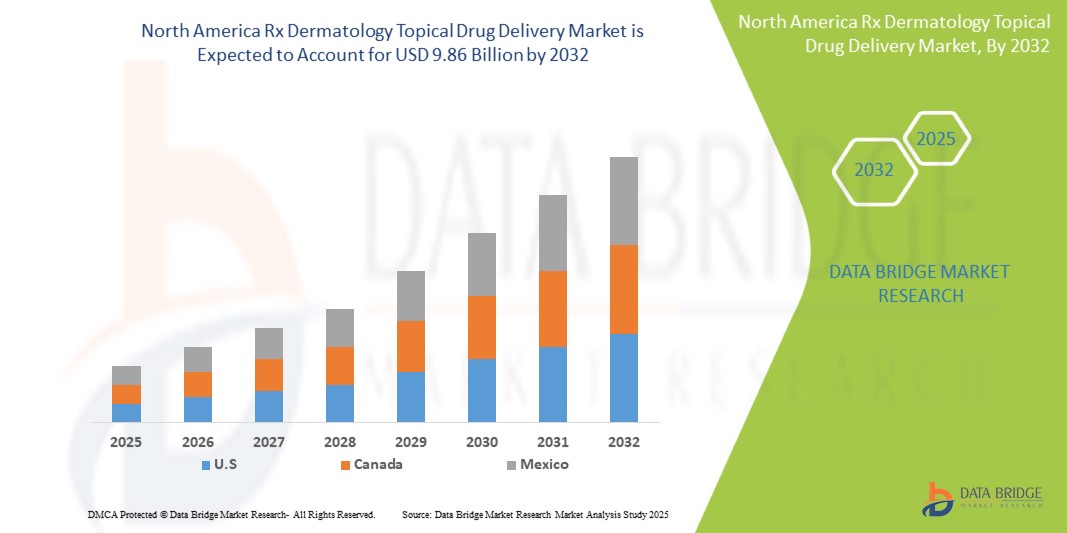
- The North America Rx Dermatology Topical Drug Delivery Market was valued at USD 5.42 billion in 2024 and is expected to reach USD 9.86 billion by 2032.
- During the forecast period of 2025 to 2032, the market is projected to grow at a CAGR of 7.8%, primarily driven by the increasing prevalence of Rx Dermatology Topical Drug Delivery , rising stress levels, and heightened awareness of mental health and sleep hygiene.
- This growth is further propelled by factors such as an aging population prone to sleep disorders, increasing adoption of pharmacological and non-pharmacological treatments, and rising demand for innovative therapies and digital sleep aids.
Rx Dermatology Topical Drug Delivery Market Analysis
- The North America Rx Dermatology Topical Drug Delivery Market is poised for substantial growth through 2032, registering a CAGR of 7.8% from 2025 to 2032.
- ChronicRx Dermatology Topical Drug Delivery and sleep disturbances, often linked to anxiety, depression, and lifestyle factors, are contributing to growing demand for both drug-based and behavioral treatments.
- The market benefits from technological advancements in digital therapeutics, increasing accessibility to cognitive behavioral therapy (CBT-I), and continued innovation in safer and more effective prescription and OTC medications.
- Growth in healthcare infrastructure, expanding telemedicine platforms, and the shift towards personalized, non-invasive treatments are expected to further strengthen market performance, especially in developing regions.
Report Scope and Rx Dermatology Topical Drug Delivery Market Segmentation
|
Attributes |
Medication-Assisted Treatment (MAT) Key Market Insights |
|
Segments Covered |
• By Drug Type: Prescription Drugs, Over-the-Counter Drugs, Herbal Remedies, Others |
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Rx Dermatology Topical Drug Delivery Market Trends
“Growing Shift Toward Targeted, Non-Invasive Dermatologic Therapies”
- A prominent trend in the North AmericaRx dermatology market is the increasing preference for targeted, non-invasive topical therapies, driven by advancements in skin absorption technologies and better understanding of dermatologic pathophysiology.
- Treatment regimens now emphasize combination therapies that pair anti-inflammatory agents, corticosteroids, and immunomodulators with innovative delivery vehicles such as liposomes, microemulsions, and transdermal patches.
- For example, products utilizing nanocarrier-based delivery for conditions like psoriasis and atopic dermatitis are gaining traction due to improved drug penetration and minimized systemic side effects.
- Digital dermatology tools, including tele-dermatology platforms and AI-powered skin imaging, are becoming essential for diagnosis and monitoring, especially in remote or underserved regions.
- Increasing consumer awareness of skin health, combined with rising rates of skin-related disorders and urban pollution, is driving demand for more effective and safer topical treatments.
Market Dynamics
Driver
“Rising Incidence of Chronic Skin Diseases and Allergic Conditions”
The North America region has witnessed a steady increase in the prevalence of dermatologic conditions such as eczema, acne, fungal infections, and contact dermatitis—conditions that significantly impact quality of life and necessitate long-term topical therapies.
Examples:
- According to a 2023 WHO report, more than 25% of the population in urbanized MEA regions report symptoms of chronic skin conditions.
- A greater focus on aesthetics, particularly among younger populations and women, is also driving demand for dermatologic interventions and specialized topical formulations.
“Expanding Access and Regulatory Reforms”
National governments are investing in dermatological care and reforming regulatory pathways to encourage innovation and reduce dependency on imports.
Examples:
- The U.S. FDA and UAE Ministry of Health have expedited the approval process for high-efficacy topical agents for inflammatory and autoimmune skin disorders.
- Government healthcare initiatives and public-private partnerships are improving access to advanced dermatology care in rural and low-income areas.
Opportunity
“Innovation in Drug Delivery Platforms and Regional Product Customization”
The market is experiencing robust growth opportunities from innovations in drug formulation, delivery mechanisms, and localization strategies tailored to the climatic and genetic skin profiles of North America populations.
Examples:
- Local manufacturers are partnering with international pharma companies to co-develop climate-adapted and pigmentation-specific topical solutions.
- Increased adoption of smart packaging (e.g., dose-metered dispensers) and mobile-linked adherence tracking for chronic skin treatments is enhancing therapy effectiveness.
- Niche opportunities exist in pediatric and geriatric dermatology, particularly for steroid-sparing regimens and moisturizers with therapeutic actives.
Restraint/Challenge
“Uneven Access and Patient Adherence Issues”
- Despite innovation, disparities in healthcare access remain a barrier in many North America countries, especially in U.S. and conflict-prone areas.
- Cultural stigma surrounding visible skin diseases often delays care-seeking behavior, impacting treatment timelines and outcomes.
- The high cost of branded topical drugs and lack of insurance coverage for dermatological care in many parts of the region limit market penetration.
- Furthermore, variations in patient adherence due to lack of education on application techniques and product storage present ongoing challenges for treatment efficacy.
Rx Dermatology Topical Drug Delivery Market Scope
The market is segmented on the basis of type, drug class, application, dosage, route of administration, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
Drug Class |
|
|
Indication |
|
|
End-Users |
|
|
Distribution Channel |
|
Rx Dermatology Topical Drug Delivery Market Regional Analysis
“North America is the Dominant Region in the Rx Dermatology Topical Drug Delivery Market”
- Countries like U.S. dominate the market due to high disposable income, advanced dermatologic infrastructure, and high awareness of cosmetic dermatology.
- North America governments are actively investing in healthcare infrastructure, including dermatology-focused clinics and mobile dermatology units.
“North Africa and Sub-Saharan Africa Show Promising Growth Potential ”
- Rising awareness campaigns, improved pharmaceutical imports, and international aid for chronic disease management are improving dermatology care.
- U.S. are emerging as key markets due to growing middle-class populations and stronger local pharmaceutical production.
- Mobile health solutions and low-cost generic formulations are expanding access across underserved populations.
Rx Dermatology Topical Drug Delivery Market Share
The competitive landscape offers detailed insights into leading market players. These include company profiles, financial metrics, R&D investments, product portfolios, North America operations, production capabilities, strategic moves, strengths, weaknesses, and their role in shaping the gastrointestinal therapeutics segment.
The Major Market Leaders Operating in the Market Include:
- GlaxoSmithKline plc
- Novartis AG
- Johnson & Johnson Services, Inc.
- Galderma S.A.
- Sanofi
- Bayer AG
- Sun Pharmaceutical Industries Ltd.
- Valeant Pharmaceuticals (Bausch Health)
- Medpharm Ltd
- Maruho Co., Ltd.
- Aspen Pharmacare
- Julphar (Gulf Pharmaceutical Industries)
- Hikma Pharmaceuticals PLC
- Cipla Ltd.
- Avalon Pharma
Latest Developments in North America Rx Dermatology Topical Drug Delivery Market
- In February 2023, Julphar launched a new range of climate-optimized topical corticosteroids in Gulf countries to address regional skin sensitivity patterns.
- In May 2022, a partnership between Medpharm and a leading Egyptian pharma manufacturer led to the introduction of nanocarrier-based antifungal creams for mass market distribution.
- In 2024, the UAE Ministry of Health introduced an AI-integrated tele-dermatology platform to facilitate rapid diagnosis and e-prescription of dermatologic therapie
- The report also emphasized the growing importance of green chemistry, continuous manufacturing technologies, and regional self-reliance in reducing supply disruptions. Increased industry focus on API traceability and quality assurance is driving a shift toward more sustainable and transparent production practices in gastrointestinal therapeutics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.