Saudi Arabia q-PCR Reagents Market Analysis and Insights
The rising public awareness regarding chronic and communicable disorders has enhanced the demand for the market. The major market players focus on various product launches and approvals during this crucial period. In addition, the increase in improved and advanced technology also contributes to the rising demand for the market.
The Saudi Arabia q-PCR Reagents market is expected to grow in the forecast period of 2023 to 2030 due to the rise in chronic diseases. The high funding for research activities by the government and other bodies is also expected to drive market growth. Along with this, manufacturers are engaged in R&D activity for launching novel products in the market. However, the high cost of the products is expected to restrain the market growth. The growing demand for better quality healthcare for communicable disorders and the rising adoption of personalized medicine is expected to boost the market growth. However, the operational barriers faced in conducting diagnostic tests are expected to challenge market growth.
Data Bridge Market Research analyzes that the Saudi Arabia q-PCR reagents market is expected to reach a value of USD 41,798.44 thousand by 2030, at a CAGR of 8.3% during the forecast period of 2023 to 2030.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015–2020) |
|
Quantitative Units |
Revenue in USD Thousand |
|
Segments Covered |
Type (Reagents and Consumables), Application (Clinical, Research, Food Product Safety Testing, Forensics, and Others), End User (Hospitals, Diagnostic Centres, Pharmaceutical and Biopharmaceutical Companies, Research and Academic Institute, Clinical Research Organization, Veterinary Labs, Forensic Laboratories, and Others), Distribution Channel (Direct Tenders, Retail Sales, and Others) |
|
Country Covered |
Saudi Arabia |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc., Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Quantabio, Sino Biological Inc., QIAGEN, TAKARA HOLDINGS INC, Enzo Biochem Inc., meridian BIOSCIENCE, Tonbo Biosciences (A subsidiary of Cytek Biosciences), Norgen Biotek Corp. |
Market Definition
q-PCR reagents are used in the simultaneous amplification and detection/quantification of nucleic acids using the polymerase chain reaction (PCR). These reagents are used in different applications such as research, diagnosis, and forensics. They are also used for the detection of viruses, genetic stability testing, and others. The main advantage of qPCR is that it allows you to determine the initial number of copies of template DNA (the amplification target sequence) with accuracy and high sensitivity over a wide dynamic range.
Saudi Arabia q-PCR Reagents Market Dynamics
This section deals with the understanding of market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Increasing Prevalence of Chronic and Communicable Diseases
Chronic diseases include cardiac diseases, cancer, and diabetes, which are the leading causes of death and disability in many countries around the globe. q-PCR reagents are essential for the diagnosis of chronic diseases such as human Immuno Deficiency Virus (HIV) and malaria. Both conditions have non-specific symptoms. The q-PCR kits and mixers contain all the necessary PCR reagents for rapid, sensitive, and reproducible real-time detection of HIV and other diseases using highly specific primer pairs.
- Increase in Geriatric Population
The geriatric population is susceptible to chronic and malignant diseases. The non-specific antibodies are more common in the elderly population. The diagnosis through q-PCR reagents is important for the elderly population as it provides early diagnosis which is crucial to prevent the extensive spread of the disease since the test is non-invasive and aids in determining antigen detection in infectious chronic diseases. Since the geriatric population is increasing, the need for q-PCR reagents will also increase.
Restraint
- Stringent Rules and Regulation
The commercialization of reagents and products by various key market players is facilitated by compliance with the regulatory frameworks established in the country. The rapid development of privacy policies and regulations being made by SFDA is restraining the market growth. The regulatory authority in charge of regulating the registration and endorsement of medical equipment, including PCR reagents, in Saudi Arabia is known as the Saudi Food and Drug Authority. The SFDA has rules for product registration, labeling, and quality control that manufacturers and distributors of PCR reagents must follow, which restrains market growth.
Opportunity
- Rise in Government Funding for Healthcare
The government, federations, and major market players are creating opportunities across developing countries. These initiatives by the players and government are helping the users to reach out to take advantage of the policies made for low-income and developing nations and making them aware of the diseases that are preventable if diagnosed earlier.
Investments in medical R&D may increase as healthcare spending rises. More studies are conducted in the areas of genetic research, illness monitoring, and diagnostics, which may increase demand for q-PCR reagents. Healthcare facilities may increase the number and type of clinical tests they offer as they develop and enhance their services, which provides the opportunity for market growth.
Challenge
- Lack of Skilled Workforce
q-PCR technology is continuously advancing and manufacturers are continuously engaged in R&D activities for launching advanced q-PCR which require highly skilled and certified professionals to operate it. Handling reagents and maintaining the PCR reactions is not a simple task. It is a vast process that requires proper knowledge, and experience so that the result conducted will be accurate and proper. However, this shortage in the professional and skilled workforce has decreased the market for q-PCR reagents.
The requirement for skilled and certified professionals is a big challenge for the market. The q-PCR is a complex process and the devices have advanced features that require skilled professionals to operate them to provide safe such as effective services to patients.
Recent Developments
- In December 2021, Thermo Fisher Scientific Inc. announced that they have completed the acquisition of PPD, Inc. a leading Saudi Arabia provider of clinical research services to the biopharma and biotech industry. This acquisition has helped the company to increase its product portfolio and sales.
- In September 2021, Agilent Technologies, Inc. announced the signing of a worldwide distribution agreement with Visiopharm, enabling Agilent to co-market Visiopharm’s portfolio of CE-IVD marked artificial intelligence (AI)-driven precision pathology software and other products. This has increased the company’s product reach.
- In September 2021, Bio-Rad Laboratories, Inc. announced the partnership with Seegene, Inc., a Saudi Arabia leader in multiplex molecular diagnostics, for the clinical development and commercialization of infectious disease molecular diagnostic products. This has increased the company’s product portfolio.
- In June 2021, Agilent Technologies, Inc. announced the launch of three InfinityLab Bio LC systems specifically developed to meet the needs of the biopharma industry which include instruments, columns, reagents, and supplies that seamlessly integrate with Agilent OpenLab and MassHunter software, and CrossLab services to maximize efficiency in biopharma labs. This has increased the company’s sales and product portfolio.
- In July 2020, Thermo Fisher Scientific Inc. announced the launch of the Applied Biosystems QuantStudio Absolute Q Digital PCR System, the first fully integrated digital PCR (dPCR) system designed to provide highly accurate and consistent results within 90 minutes. This has increased the company’s revenue and product portfolio.
Saudi Arabia q-PCR Reagents Market Scope
The Saudi Arabia q-PCR reagents market is segmented into four notable segments based on type, application, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
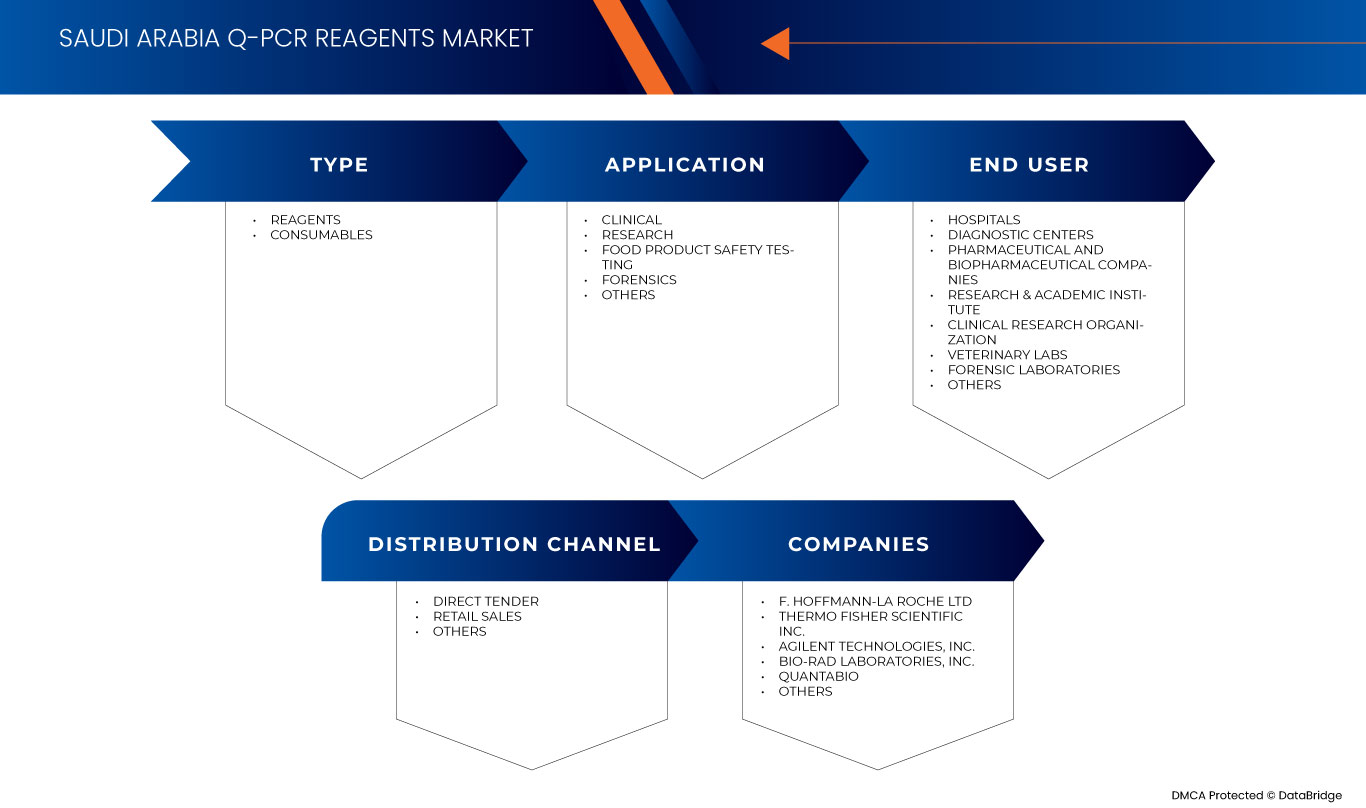
Type
- Reagents
- Consumables
On the basis of type, the market is segmented into reagents and consumables.
Application
- Clinical
- Research
- Food Product Safety Testing
- Forensics
- Others
On the basis of application, the market is segmented into clinical, research, food product safety testing, forensics, and others.
End User
- Hospitals
- Diagnostic Centres
- Pharmaceutical and Biopharmaceutical Companies
- Research & Academic Institute
- Clinical Research Organization
- Veterinary Labs
- Forensic Laboratories
- Others
On the basis of end user, the market is segmented into hospitals, diagnostic centres, pharmaceutical and biopharmaceutical companies, research & academic institute, clinical research organization, veterinary labs, forensic laboratories, and others.
Distribution Channel
- Direct Tender
- Retail Sales
- Others
On the basis of distribution channel, the market is segmented into direct tender, retail sales, and others.
Competitive Landscape and Saudi Arabia q-PCR Reagents Market Share Analysis
The Saudi Arabia q-PCR reagents market competitive landscape provides details of competitors. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, and technology lifeline curve. The above data points provided are only related to the company’s focus on the Saudi Arabia q-PCR reagents market.
Some of the major market players operating in the Saudi Arabia q-PCR reagents market are F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc., Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Quantabio, Sino Biological Inc., QIAGEN, TAKARA HOLDINGS INC, Enzo Biochem Inc., meridian BIOSCIENCE, Tonbo Biosciences (A subsidiary of Cytek Biosciences), Norgen Biotek Corp., among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF SAUDI ARABIA Q-PCR REAGENTS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCTS LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GLOBAL QPCR REAGENTS MARKET, PRICING ANALYSIS
5 COST ANALYSIS BREAKDOWN
6 HEALTHCARE ECONOMY
7 INDUSTRY INSIGHTS
8 INNOVATION TRACKER AND STRATEGIC ANALYSIS
9 TECHNOLOGY ROADMAP
10 VALUE CHAIN ANALYSIS
11 REGULATIONS
12 MARKET OVERVIEW
12.1 DRIVERS
12.1.1 INCREASING PREVALENCE OF CHRONIC AND COMMUNICABLE DISEASES
12.1.2 INCREASE IN GERIATRIC POPULATION
12.1.3 INCREASE IN EARLY DIAGNOSIS RATE
12.1.4 INCREASE IN ADVANCED TECHNOLOGY
12.2 RESTRAINTS
12.2.1 STRINGENT RULES AND REGULATIONS
12.2.2 HIGH COST OF PRODUCT
12.3 OPPORTUNITIES
12.3.1 RISE IN GOVERNMENT FUNDING FOR HEALTHCARE
12.3.2 RISE IN HEALTHCARE EXPENDITURE
12.4 CHALLENGES
12.4.1 LACK OF SKILLED WORKFORCE
12.4.2 OPERATIONAL BARRIERS FACED IN CONDUCTING DIAGNOSTIC TESTS
13 SAUDI ARABIA QPCR REAGENTS MARKET, BY TYPE
13.1 OVERVIEW
13.2 REAGENTS
13.2.1 MASTER MIX
13.2.1.1 DNA
13.2.1.2 RNA
13.2.2 PRIMERS AND DNTPS
13.2.3 ASSAY
13.2.4 NUCLEIC ACID GEL STAIN
13.2.5 DNASE BUFFER
13.2.6 OTHERS
13.2. CONSUMABLES
13.2.1. PIPETTE TIPS
13.2.2. TUBES
13.2.3. PLATES
13.2.3.1. STANDARD
13.2.3.2. CUSTOM
13.2.4. SEALING FOILS
13.2.5. TUBE STRIPS
13.2.6. OTHERS
14 SAUDI ARABIA QPCR REAGENTS MARKET, BY APPLICATION
14.2. OVERVIEW
14.3. CLINICAL
13.3 HUMAN DIAGNOSTICS
14.3.1.1. HOSPITALS
14.3.1.2. DIAGNOSTIC CENTERS
14.3.1.3. PATHOLOGY LABS
14.3.1.4. OTHERS
13.4 VETERINARY DIAGNOSTICS
14.3.1.5. VETERINARY HOSPITALS
14.3.1.6. DIAGNOSTIC CENTERS
14.3.1.7. VETERINARY LABORATORY
14.3.1.8. VETERINARY CLINICS
14.4. RESEARCH
14.4.1. GENE EXPRESSION ANALYSIS
14.4.1.1. RESEARCH & ACADEMIC INSTITUTES
14.4.1.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.1.3. OTHERS
14.4.2. GENOTYPING
14.4.2.1. RESEARCH & ACADEMIC INSTITUTES
14.4.2.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.2.3. OTHERS
14.4.3. MICROBE AND PATHOGEN DETECTION
14.4.3.1. RESEARCH & ACADEMIC INSTITUTES
14.4.3.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.3.3. OTHERS
14.4.4. SINGLE NUCLEOTIDE POLYMORPHISM (SNP) DETECTION
14.4.4.1. RESEARCH & ACADEMIC INSTITUTES
14.4.4.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.4.3. OTHERS
14.4.5. DRUG DEVELOPMENT
14.4.5.1. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.5.2. CLINICAL RESEARCH ORGANIZATION
14.4.5.3. RESEARCH & ACADEMIC INSTITUTES
14.4.5.4. OTHERS
14.4.6. DRUG DISCOVERY
14.4.6.1. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.6.2. CLINICAL RESEARCH ORGANIZATION
14.4.6.3. RESEARCH & ACADEMIC INSTITUTES
14.4.6.4. OTHERS
14.4.7. OTHERS
14.5. FOOD PRODUCT SAFETY TESTING
14.5.1. FOOD REGULATORY AGENCIES
14.5.2. FOOD TESTING LABORATORIES
14.5.3. FOOD COMPANIES
14.5.4. OTHERS
14.6. FORENSICS
14.6.1. PRIVATE FORENSIC LABORATORIES
14.6.2. PUBLIC FORENSIC LABORATORIES
14.7. OTHERS
15 SAUDI ARABIA QPCR REAGENTS MARKET, BY END USER
15.2. OVERVIEW
15.3. HOSPITALS
15.4. DIAGNOSTIC CENTERS
15.5. PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES
15.6. RESEARCH & ACADEMIC INSTITUTE
15.7. CLINICAL RESEARCH ORGANIZATION
15.8. VETERINARY LABS
15.9. FORENSIC LABORATORIES
15.10. OTHERS
16 SAUDI ARABIA QPCR REAGENTS MARKET, BY DISTRIBUTION CHANNEL
16.2. OVERVIEW
16.3. DIRECT TENDER
16.4. RETAIL SALES
16.5. OTHERS
17 SAUDI ARABIA QPCR REAGENTS MARKET: COMPANY LANDSCAPE
17.2. COMPANY SHARE ANALYSIS: SAUDI ARABIA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.2. F. HOFFMANN-LA ROCHE LTD
19.2.1. COMPANY SNAPSHOT
19.2.2. REVENUE ANALYSIS
19.2.3. PRODUCT PORTFOLIO
19.2.4. RECENT DEVELOPMENT
19.3. THERMO FISHER SCIENTIFIC INC.
19.3.1. COMPANY SNAPSHOT
19.3.2. REVENUE ANALYSIS
19.3.3. PRODUCT PORTFOLIO
19.3.4. RECENT DEVELOPMENTS
19.4. AGILENT TECHNOLOGIES, INC.
19.4.1. COMPANY SNAPSHOT
19.4.2. REVENUE ANALYSIS
19.4.3. PRODUCT PORTFOLIO
19.4.4. RECENT DEVELOPMENTS
19.4.4.1. DISTRIBUTION AGREEMENT
19.4.4.2. PRODUCT LAUNCH
19.5. BIO-RAD LABORATORIES, INC.
19.5.1. COMPANY SNAPSHOT
19.5.2. REVENUE ANALYSIS
19.5.3. PRODUCT PORTFOLIO
19.5.4. RECENT DEVELOPMENTS
19.6. QUANTABIO
19.6.1. COMPANY SNAPSHOT
19.6.2. PRODUCT PORTFOLIO
19.6.3. RECENT DEVELOPMENT
19.7. ENZO BIOCHEM INC
19.7.1. COMPANY SNAPSHOT
19.7.2. REVENUE ANALYSIS
19.7.3. PRODUCT PORTFOLIO
19.7.4. RECENT DEVELOPMENT
19.8. MERIDIAN BIOSCIENCE
19.8.1. COMPANY SNAPSHOT
19.8.2. PRODUCT PORTFOLIO
19.8.3. RECENT DEVELOPMENTS
19.9. NORGEN BIOTEK CORP
19.9.1. COMPANY SNAPSHOT
19.9.2. PRODUCT PORTFOLIO
19.9.3. RECENT DEVELOPMENT
19.10. QIAGEN
19.10.1. COMPANY SNAPSHOT
19.10.2. REVENUE ANALYSIS
19.10.3. PRODUCT PORTFOLIO
19.10.4. RECENT DEVELOPMENTS
19.11. SINO BIOLOGICAL INC.
19.11.1. COMPANY SNAPSHOT
19.11.2. PRODUCT PORTFOLIO
19.11.3. RECENT DEVELOPMENT
19.12. TAKARA HOLDINGS INC
19.12.1. COMPANY SNAPSHOT
19.12.2. REVENUE ANALYSIS
19.12.3. PRODUCT PORTFOLIO
19.12.4. RECENT DEVELOPMENTS
19.13. TONBO BIOSCIENCES (A SUBSIDIARY OF CYTEK BIOSCIENCES)
19.13.1. COMPANY SNAPSHOT
19.13.2. PRODUCT PORTFOLIO
19.13.3. RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
List of Table
TABLE 1 THE FOLLOWING ARE THE PRICES OF PRODUCTS (CONSUMABLES AND REAGENTS) OFFERED BY THE COMPANIES
TABLE 2 MEDICAL DEVICE CLASSIFICATION SYSTEM:
TABLE 3 APPROVAL PROCESS OF MEDICAL DEVICES:
TABLE 4 SAUDI ARABIA QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 5 SAUDI ARABIA REAGENTS IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 SAUDI ARABIA MASTER MIX IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 7 SAUDI ARABIA CONSUMABLES IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 8 SAUDI ARABIA PLATES IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 SAUDI ARABIA QPCR REAGENTS MARKET, BY APPLICATION, 2021-2030 (USD THOUSAND)
TABLE 10 SAUDI ARABIA CLINICAL IN QPCR REAGENTS MARKET, BY APPLICATION, 2021-2030 (USD THOUSAND)
TABLE 11 SAUDI ARABIA HUMAN DIAGNOSTICS IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 12 SAUDI ARABIA VETERINARY DIAGNOSTICS IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 13 SAUDI ARABIA RESEARCH IN QPCR REAGENTS MARKET, BY APPLICATION, 2021-2030 (USD THOUSAND)
TABLE 14 SAUDI ARABIA GENE EXPRESSION ANALYSIS IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 15 SAUDI ARABIA GENOTYPING IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 16 SAUDI ARABIA MICROBE AND PATHOGEN DETECTION IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 17 SAUDI ARABIA SINGLE NUCLEOTIDE POLYMORPHISM (SNP) DETECTION IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 18 SAUDI ARABIA DRUG DEVELOPMENT IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 19 SAUDI ARABIA DRUG DISCOVERY IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 20 SAUDI ARABIA FOOD PRODUCT SAFETY TESTING IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 21 SAUDI ARABIA FORENSIC IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 22 SAUDI ARABIA QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 23 SAUDI ARABIA QPCR REAGENTS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
List of Figure
FIGURE 1 SAUDI ARABIA Q-PCR REAGENTS MARKET: SEGMENTATION
FIGURE 2 SAUDI ARABIA Q-PCR REAGENTS MARKET: DATA TRIANGULATION
FIGURE 3 SAUDI ARABIA Q-PCR REAGENTS MARKET: DROC ANALYSIS
FIGURE 4 SAUDI ARABIA Q-PCR REAGENTS MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 SAUDI ARABIA Q-PCR REAGENTS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 SAUDI ARABIA Q-PCR REAGENTS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 SAUDI ARABIA Q-PCR REAGENTS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 SAUDI ARABIA Q-PCR REAGENTS MARKET: MARKET END USER GRID
FIGURE 9 SAUDI ARABIA Q-PCR REAGENTS MARKET: SEGMENTATION
FIGURE 10 INCREASING GERIATRIC POPULATION AND INCREASE IN EARLY DIAGNOSIS RATE IS EXPECTED TO DRIVE THE GROWTH OF THE SAUDI ARABIA Q-PCR REAGENTS MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 11 TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE SAUDI ARABIA Q-PCR REAGENTS MARKET FROM 2023 TO 2030
FIGURE 12 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF SAUDI ARABIA Q-PCR REGANETS MARKET
FIGURE 13 SAUDI ARABIA QPCR REAGENTS MARKET: BY TYPE, 2022
FIGURE 14 SAUDI ARABIA QPCR REAGENTS MARKET: BY APPLICATION, 2022
FIGURE 15 SAUDI ARABIA QPCR REAGENTS MARKET: BY END USER, 2022
FIGURE 16 SAUDI ARABIA QPCR REAGENTS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 17 SAUDI ARABIA Q-PCR REAGENTS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.