Global Viral Vector Purification Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
813.03 Million
USD
2,387.52 Million
2024
2032
USD
813.03 Million
USD
2,387.52 Million
2024
2032
| 2025 –2032 | |
| USD 813.03 Million | |
| USD 2,387.52 Million | |
|
|
|
|
Global Viral Vector Purification Market, By Product and Services (Products and Services), Type (Retroviral Vectors, Vaccine Virus, Adenoviral Vectors, Adeno-Associated Viral Vectors, Lentivirus and Other), Workflow (Upstream Processing and Downstream Processing), Purification Technique (Density-Gradient Ultracentrifugation, Ultrafiltration, Precipitation, Two-Phase Extraction Systems and Chromatography), Scale of Operation (Preclinical / Clinical and Commercial), Delivery Method (In Vivo and Ex Vivo), Disease Indication (Cancer, Genetic Disorders, Infectious Diseases, Veterinary Disease and Other), Application (Antisense And RNAi, Gene Therapy, Cell Therapy and Vaccinology), End User (Biotechnology Companies, Pharmaceutical Companies, Contract Research Organizations, Contract Development and Manufacturing Organization (CDMO) and Academic/ Research Institutes) – Industry Trends and Forecast to 2031.
Viral Vector Purification Market Analysis and Size
The viral vector purification market is advancing rapidly with innovative technologies enhancing purification processes. This evolution includes novel chromatography techniques, membrane-based systems, and scalable solutions for large-scale viral vector production. These advancements contribute to the efficient isolation and purification of viral vectors, crucial for gene therapy and vaccine development applications.
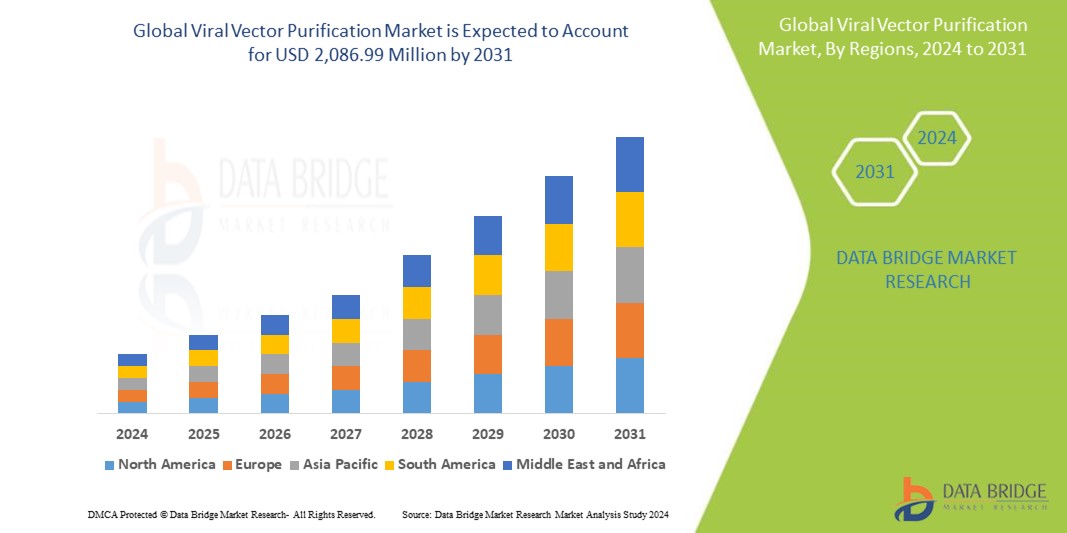
Data Bridge Market Research analyses that the global viral vector purification market, which was USD 721.42 million in 2023, is expected to reach USD 2,086.99 million by 2031, and is expected to undergo a CAGR of 14.2% during the forecast period of 2024 to 2031. This indicates that the market value. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Viral Vector Purification Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2024 to 2031 |
|
Base Year |
2023 |
|
Historic Years |
2022 (Customizable to 2016-2021) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product and Services (Products and Services), Type (Retroviral Vectors, Vaccine Virus, Adenoviral Vectors, Adeno-Associated Viral Vectors, Lentivirus and Other), Workflow (Upstream Processing and Downstream Processing), Purification Technique (Density-Gradient Ultracentrifugation, Ultrafiltration, Precipitation, Two-Phase Extraction Systems and Chromatography), Scale of Operation (Preclinical / Clinical and Commercial), Delivery Method (In Vivo and Ex Vivo), Disease Indication (Cancer, Genetic Disorders, Infectious Diseases, Veterinary Disease and Other), Application (Antisense And RNAi, Gene Therapy, Cell Therapy and Vaccinology), End User (Biotechnology Companies, Pharmaceutical Companies, Contract Research Organizations, Contract Development and Manufacturing Organization (CDMO) and Academic/ Research Institutes) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Market Players Covered |
Applied Biological Materials Inc. (Canada), Creative Biolabs (U.S.), Bio-Rad Laboratories, Inc. (U.S.), SIRION BIOTECH GmbH (Germany), Merck KGaA (Gemrany), FUJIFILM Diosynth Biotechnologies (U.S.), Batavia Biosciences B.V. (Netherlands), Agilent Technologies, Inc. (U.S.), Addgene (U.S.), IDT Biologika (Germany), ProBioGen AG (Germany), Takara Bio Inc. (Japan), Thermo Fisher Scientific Inc. (U.S.), Waisman Biomanufacturing (U.S.), Creative Biogene (U.S.), Aldevron (U.S.), uniQure N.V. (Netherlands), Cytiva (U.S.) and Sartorius BIA Separations (Solvenia) |
|
Market Opportunities |
|
Market Definition
Viral vector purification is a process aimed at removing impurities and concentrating viral vectors used in gene therapy or vaccine development. It typically involves techniques such as ultracentrifugation, chromatography, and filtration to isolate and purify the viral particles, ensuring safety and efficacy in biomedical applications.
Global Viral Vector Purification Market Dynamics
Drivers
- Increasing Demand for Gene Therapy for Chronic Illnesses
The rising incidence of genetic disorders and chronic illnesses is driving a surge in demand for gene therapy. This heightened need is fueling the viral vector purification technologies market, playing a crucial role in addressing a spectrum of medical conditions through efficient isolation and purification processes for gene therapy applications.
- Advancements in Biotechnology Improving Efficiency and Scalability
Biotechnological advancements are revolutionizing viral vector production, elevating efficiency and scalability. Continuous innovations in bioprocessing techniques and vector engineering are propelling the demand for sophisticated purification methods. This dynamic landscape fosters the development of cutting-edge technologies, ensuring the purification of high-quality viral vectors critical for the success of gene therapies and vaccines.
Opportunities
- Increasing Clinical Trials Demands for Purified Vectors
The surge in clinical trials for viral vector-based therapies signifies a growing demand for purified vectors, presenting a substantial opportunity for companies specializing in viral vector purification. As the pipeline of therapeutic candidates expands, there's a heightened need for efficient and scalable purification technologies, driving market growth and innovation.
- Emerging Applications into Immunotherapy and Vaccine Development
The growing utilization of viral vectors in immunotherapy and vaccine development offers a lucrative avenue for purification technologies. The escalating need for reliable and effective vectors in these domains propels market expansion and spurs innovation, positioning purification technologies as indispensable assets in advancing therapeutic and preventive interventions.
Restraints/Challenges
- High Purification Costs of Therapies Restricts the Affordability
High purification costs pose a significant barrier to market access for therapies, especially in resource-constrained healthcare settings. The financial burden restricts the affordability and availability of viral vector-based treatments, hindering patient access and adoption, exacerbating healthcare disparities, and impeding market growth.
- Complexity in Ensuring Stability of Viral Vector
Viral vector stability poses a significant challenge in the market, as maintaining the integrity of these vectors during purification is complex. Shear forces, temperature variations, and exposure to specific chemicals can compromise their structural integrity, potentially reducing therapeutic efficacy and overall product quality. This instability necessitates rigorous optimization and innovation in purification processes to mitigate these risks and ensure the success of viral vector-based therapies.
This global viral vector purification market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global viral vector purification market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In January 2021, Thermo Fisher Scientific Inc. finalized the acquisition of Novasep's viral vector manufacturing business in Belgium. This strategic move enables Thermo Fisher to enhance its market diversification efforts, strengthening its position in the rapidly expanding field of viral vector production for gene therapy and vaccine development
- In January 2021, Aldevron expanded its product portfolio by launching rep/cap plasmids pALD-AAV2, AAV5, and AAV6 to support AAV viral vector manufacturing. This addition has sparked increased demand and sales, positioning the company for future revenue growth in the gene therapy and vaccine development markets
- In September 2020, Waisman Biomanufacturing formed a strategic partnership with California-based biotech firm GigaGen to develop a novel drug for COVID-19 treatment and prevention. This collaboration is poised to enhance Waisman Biomanufacturing's role in drug development and testing, amplifying awareness of its services within the pharmaceutical market
Global Viral Vector Purification Market Scope
The global viral vector purification market is segmented on the basis of product and services, type, workflow, purification technique, scale of operation, delivery method, disease indication, application and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product and Services
- Product
- Kit
- Prepacked
- Column
- Resin
- Cassette
- Capsule
- Reagent
- Services
Type
- Retroviral Vectors
- Vaccine Virus
- Adenoviral Vectors
- Adeno-Associated Viral Vectors
- Lentivirus
- Other
Workflow
- Upstream Processing
- Downstream Processing
Purification Technique
- Density-Gradient Ultracentrifugation
- Ultrafiltration
- Precipitation
- Two-Phase Extraction Systems
- Chromatography
Scale of Operation
- Preclinical / Clinical
- Commercial
Delivery Method
- In Vivo
- Ex Vivo
Disease Indication
- Cancer
- Genetic Disorders
- Infectious Diseases
- Veterinary Disease
- Other
Application
- Antisense and RNAi
- Gene Therapy
- Cell Therapy
- Vaccinology
End User
- Biotechnology Companies
- Pharmaceutical Companies
- Contract Research Organizations
- Contract Development and Manufacturing Organization (CDMO)
- Academic/ Research Institutes
Global Viral Vector Purification Market Regional Analysis/Insights
The global viral vector purification market is analysed and market size insights and trends are provided by country, product and services, type, workflow, purification technique, scale of operation, delivery method, disease indication, application and end user as referenced above.
The countries covered in the global viral vector purification market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to dominate the viral vector purification market, driven by widespread adoption among patients. The U.S. leads this growth within the region, propelled by a significant presence of key market players. This dominance underscores North America's pivotal role in advancing viral vector purification technologies and therapies.
Europe is expected to dominate the viral vector purification market from 2024 to 2031, driven by its advanced healthcare infrastructure and rising healthcare investments. Germany emerges as a dominant force within the Europe sector, propelled by continuous advancements in viral vector purification technologies.
Asia-Pacific is expected to dominate the viral vector purification market, exhibiting the fastest compound annual growth rate (CAGR) in the forecast period. The region's growing population and rising healthcare expenditure drive the escalating demand. Japan, a frontrunner in utilizing advanced viral vector purification products, is anticipated to lead the market within Asia-Pacific.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The global viral vector purification market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for global viral vector purification market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the global viral vector purification market. The data is available for historic period 2016-2021.
Competitive Landscape and Global Viral Vector Purification Market Share Analysis
The global viral vector purification market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global viral vector purification market.
Some of the major players operating in the global viral vector purification market are:
- Applied Biological Materials Inc. (Canada)
- Creative Biolabs (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- SIRION BIOTECH GmbH (Germany)
- Merck KGaA (Gemrany)
- FUJIFILM Diosynth Biotechnologies (U.S.)
- Batavia Biosciences B.V. (Netherlands)
- Agilent Technologies, Inc. (U.S.)
- Addgene (U.S.)
- IDT Biologika (Germany)
- ProBioGen AG (Germany)
- Takara Bio Inc. (Japan)
- Thermo Fisher Scientific Inc. (U.S.)
- Waisman Biomanufacturing (U.S.)
- Creative Biogene (U.S.)
- Aldevron (U.S.)
- uniQure N.V. (Netherlands)
- Cytiva (U.S.)
- Sartorius BIA Separations (Solvenia)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.