Gsa And Benelux Point Care Testing Poct Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
3.63 Billion
USD
9.07 Billion
2024
2032
USD
3.63 Billion
USD
9.07 Billion
2024
2032
| 2025 –2032 | |
| USD 3.63 Billion | |
| USD 9.07 Billion | |
|
|
|
|
GSA и рынок тестирования в пунктах оказания медицинской помощи (POCT) стран Бенилюкса, по типу продукта (продукты для мониторинга глюкозы, продукты для тестирования на инфекционные заболевания, продукты для кардиометаболического мониторинга, продукты для тестирования на беременность и фертильность, продукты для гематологического тестирования, продукты для мониторинга коагуляции, продукты для тестирования на наркотики (DOA), продукты для анализа мочи, продукты для тестирования холестерина, продукты для тестирования на маркеры опухолей/рака, продукты для тестирования кала на скрытую кровь и другие), по платформе (тесты с латеральным потоком/иммунохроматографические тесты, иммуноанализы, тест-полоски, молекулярная диагностика, клинические химические анализы, микрофлюидика, гематология, другие), по применению (глюкоза в крови, инфекционные заболевания, мониторинг основных показателей жизнедеятельности, кардиомониторинг, коагуляция, гематология, неинвазивный мониторинг SpO2, анализ крови Переливание, неинвазивный мониторинг PCO2, анализ цельной крови и другие), по способу назначения (тестирование по рецепту и тестирование без рецепта), по конечному пользователю (больницы, уход на дому, клиники, лаборатории, диагностические центры, патологические лаборатории, амбулаторные хирургические центры, центры ухода за пожилыми людьми и другие), по каналу сбыта (прямой тендер, розничные продажи, онлайн-продажи и другие) - отраслевые тенденции и прогноз до 2032 г.
Размер рынка тестирования в местах оказания медицинской помощи (POCT)
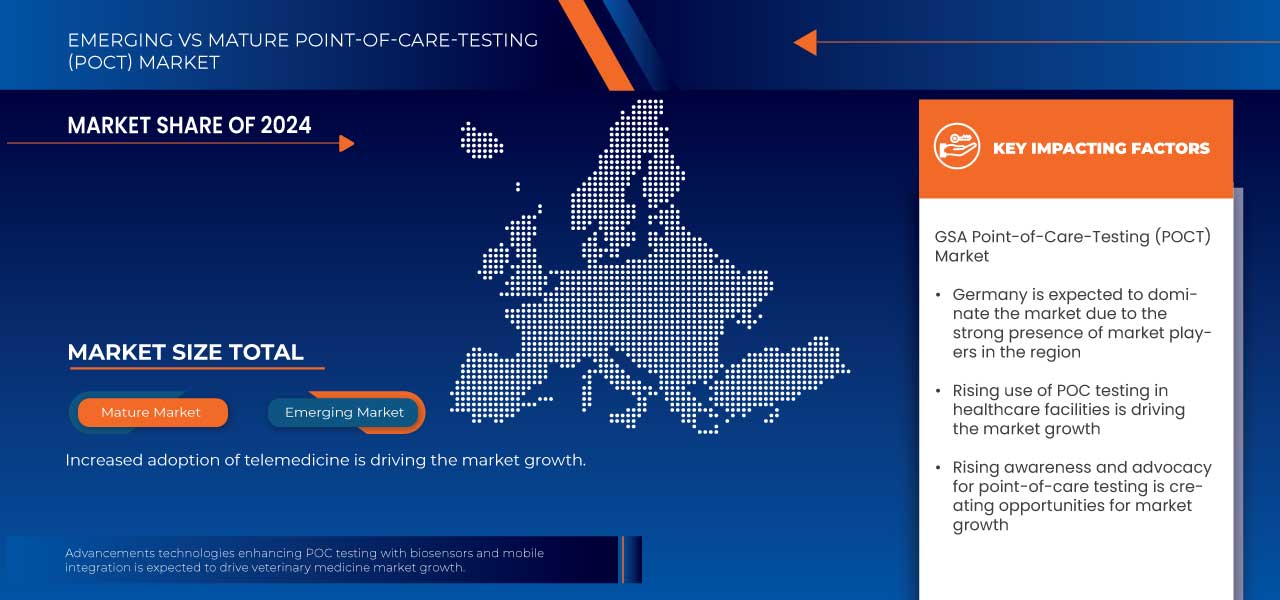
- Объем рынка тестирования в пунктах оказания медицинской помощи (POCT) GSA оценивался в 3,63 млрд долларов США в 2024 году и, как ожидается , достигнет 9,07 млрд долларов США к 2032 году при среднегодовом темпе роста 12,1% в течение прогнозируемого периода.
- Объем рынка тестирования в пунктах оказания медицинской помощи (POCT) в странах Бенилюкса оценивался в 882 млн долларов США в 2024 году и, как ожидается , достигнет 1819 млн долларов США к 2032 году при среднегодовом темпе роста 9,5% в прогнозируемый период.
- Этот рост обусловлен такими факторами, как растущий спрос на быстрые результаты диагностики, рост распространенности хронических и инфекционных заболеваний и достижения в области портативных диагностических технологий.
Анализ рынка тестирования в точках оказания медицинской помощи (POCT)
- Устройства Point-of-Care Testing (POCT) играют важную роль в предоставлении быстрых диагностических результатов в месте оказания помощи пациенту или рядом с ним, значительно улучшая принятие клинических решений в различных медицинских учреждениях. Эти тесты широко используются при лечении хронических заболеваний, инфекционных заболеваний и неотложной помощи
- Спрос на решения POCT в регионах GSA и Бенилюкса обусловлен в первую очередь растущим бременем хронических заболеваний, ростом численности пожилого населения и сильным стремлением к децентрализованным и ориентированным на пациента моделям здравоохранения.
- Ожидается, что Германия будет доминировать на рынке POCT с долей 28,04% в регионе благодаря своей хорошо развитой инфраструктуре здравоохранения, высокой пропускной способности диагностики и внедрению передовых медицинских технологий.
- Прогнозируется, что в странах Бенилюкса будет наблюдаться сильный рост рынка, обусловленный благоприятными правительственными инициативами, ростом расходов на здравоохранение и более широким внедрением POCT как в клинических условиях, так и в условиях домашнего ухода.
- Ожидается, что сегмент тестирования уровня глюкозы в крови с долей 34,08% станет лидером на рынке POCT в регионе, что обусловлено высокой распространенностью диабета и спросом на инструменты мониторинга в режиме реального времени, которые расширяют возможности пациентов в управлении заболеванием.
Область применения отчета и сегментация рынка тестирования в местах оказания медицинской помощи (POCT)
|
Атрибуты |
Ключевые сведения о рынке офтальмологических операционных микроскопов |
|
Охваченные сегменты |
|
|
Страны, охваченные |
ГСА
БЕНИЛЮКС
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают анализ импорта и экспорта, обзор производственных мощностей, анализ потребления продукции, анализ ценовых тенденций, сценарий изменения климата, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья и расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка тестирования в точках оказания медицинской помощи (POCT)
«Достижения в области быстрой диагностики и децентрализованного тестирования в странах GSA и Бенилюкса»
- Одной из важных тенденций, определяющих рынок POCT в регионах GSA и Бенилюкс, является растущая интеграция цифровой связи, миниатюрных датчиков и возможностей мультиплексного тестирования в устройствах для оказания медицинской помощи.
- Эти инновации повышают скорость и точность диагностики, позволяя поставщикам медицинских услуг принимать своевременные решения, особенно в условиях чрезвычайной ситуации, при лечении хронических заболеваний и скрининге инфекционных заболеваний.
- Например, системы POCT, оснащенные беспроводной передачей данных и интеграцией электронных медицинских карт (ЭМК), все чаще используются в учреждениях первичной медико-санитарной помощи по всей Германии и Нидерландам для оптимизации мониторинга пациентов и сокращения количества посещений больниц.
- Эти достижения революционизируют децентрализованную диагностику, поддерживают модели оказания медицинской помощи, основанные на ценностях, и ускоряют переход к здравоохранению, ориентированному на пациента, в регионах GSA и Бенилюкса.
- Растущее внимание к профилактической медицине в сочетании с мощной государственной поддержкой раннего выявления заболеваний продолжает стимулировать спрос на решения POCT нового поколения с улучшенными аналитическими характеристиками и удобством использования.
Динамика рынка тестирования в местах оказания медицинской помощи (POCT)
Водитель
«Растущее использование POC-тестирования в медицинских учреждениях»
- Растущее использование тестирования в местах оказания медицинской помощи (POC) в медицинских учреждениях кардинально меняет подходы поставщиков медицинских услуг к диагностике и лечению заболеваний.
- С ростом потребности в быстрых и точных результатах диагностики специалисты здравоохранения обращаются к POC-тестированию для улучшения ухода за пациентами. Этот сдвиг особенно заметен в отделениях неотложной помощи, амбулаторных клиниках и диагностических центрах, где быстрое принятие решений имеет решающее значение
- Тестирование в пунктах оказания медицинской помощи позволяет поставщикам медицинских услуг получать результаты немедленно по месту оказания медицинской помощи, избавляя пациентов от необходимости ждать результатов лабораторных исследований и значительно сокращая время диагностики и лечения.
- Кроме того, тесты POC очень портативны и удобны в использовании, что делает их идеальными для использования в различных медицинских учреждениях: от сельских клиник до крупных больниц.
- Рост распространенности хронических заболеваний и растущий спрос на эффективные решения в области здравоохранения еще больше способствуют внедрению POC-тестирования, поскольку оно позволяет лучше контролировать заболевания, особенно такие состояния, как диабет, сердечно-сосудистые заболевания и инфекционные заболевания.
- Поскольку медицинские учреждения все чаще внедряют эти технологии в свои рабочие процессы, спрос на тестирование в местах оказания медицинской помощи продолжает расти, выступая ключевым фактором расширения рынка.
Например,
- В марте 2024 года, по данным NCBI, быстрое время выполнения POCT улучшает принятие клинических решений, сокращает время управления и повышает эффективность сортировки, особенно в отделениях неотложной помощи (ED). Его гибкость распространяется на догоспитальные учреждения, полевые госпитали, а также во время стихийных бедствий, массовых собраний или в переполненных районах.
- В ноябре 2024 года, согласно статье, опубликованной OBJECTIVE Investment Banking & Valuation, переход к тестированию в месте оказания медицинской помощи (POCT) произвел революцию в здравоохранении, перенеся диагностику непосредственно в место оказания медицинской помощи пациентам. Его скорость, удобство и эффективность делают его бесценным в экстренных, амбулаторных и удаленных условиях. Поскольку спрос на быструю диагностику растет
- Растущее внедрение тестирования Point-of-Care (POC) в медицинских учреждениях трансформирует диагностику и лечение заболеваний. С потребностью в быстрых и точных результатах поставщики медицинских услуг используют тестирование POC для улучшения ухода за пациентами
Возможность
«Повышение осведомленности и пропаганда тестирования в местах оказания медицинской помощи»
- По мере того, как специалисты в области здравоохранения, политики и широкая общественность становятся более осведомленными о преимуществах POCT, таких как быстрые результаты, улучшенное ведение пациентов, сокращение сроков выполнения и потенциал для децентрализованного тестирования, растет спрос на эти решения.
- Пропагандистские усилия со стороны групп пациентов, профессиональных организаций и отраслевых органов еще больше подчеркивают ценность POCT в различных клинических условиях, включая отделения неотложной помощи, первичную медицинскую помощь и даже в общественных учреждениях.
- Это возросшее понимание и поддержка трансформируются в большую готовность инвестировать и интегрировать POCT в существующие рабочие процессы здравоохранения, расширяя размер рынка и охват за счет более широкого внедрения и использования.
Например,
- В апреле 2023 года NCBI заявил, что подавляющее большинство немецких врачей общей практики (93%) считают тесты по месту оказания медицинской помощи (POCT) ценными диагностическими инструментами в своей практике. В качестве основных преимуществ использования POCT они в первую очередь назвали возможность принимать немедленные решения по лечению пациентов и повышение диагностической уверенности.
- Согласно статье под названием «Тестирование в месте оказания первой помощи в первичной медицинской помощи в Нидерландах», опубликованной Open Repository, специалисты здравоохранения все чаще используют диагностические тесты в месте оказания первой помощи (POC) для принятия терапевтических решений. Основным преимуществом этих тестов является их способность быстро давать результаты непосредственно в месте нахождения пациента или рядом с ним.
- Более того, эта повышенная осведомленность и пропаганда могут привести к благоприятным изменениям политики и инициативам финансирования, которые поддерживают более широкое внедрение POCT. Правительства и системы здравоохранения в GSA и Бенилюксе, признавая потенциал POCT для повышения эффективности и доступа к диагностике, могут выделить больше ресурсов на внедрение этих технологий
- Пропаганда также может повлиять на регулирующие органы, чтобы оптимизировать процессы одобрения инновационных устройств POCT, ускоряя их выход на рынок. Это создает более благоприятную среду для роста рынка, поощряя как устоявшихся игроков, так и новых участников инвестировать в разработку и внедрение передовых решений POCT для удовлетворения растущего спроса
Сдержанность/Вызов
«Проблемы безопасности и конфиденциальности данных»
- Поскольку все больше устройств POCT интегрируются с цифровыми платформами, сбор, передача и хранение конфиденциальной информации о пациентах повышают риски несанкционированного доступа и утечки данных. Медицинские данные, которые включают персональные медицинские данные, требуют строгой защиты в соответствии с такими законами, как HIPAA и GDPR, что создает дополнительные сложности для производителей и поставщиков медицинских услуг.
- Отсутствие надежных систем безопасности и протоколов шифрования в некоторых устройствах POCT еще больше усиливает эти опасения.
- Более того, нежелание пациентов делиться своими медицинскими данными из-за рисков конфиденциальности может помешать широкому внедрению, особенно в регионах, где доверие к цифровым медицинским решениям ограничено. Текущая разработка безопасных систем, соответствующих требованиям конфиденциальности, имеет решающее значение, но текущие проблемы сдерживают темпы инноваций и внедрения на рынке GSA и Benelux POCT
Например,
- В июле 2021 года, согласно блогу, опубликованному MDPI, одним из важных аспектов, которые следует учитывать в отношении рисков кибербезопасности и конфиденциальности, являются системы медицинского обслуживания Point-of-Care (POC), которые широко используются в больницах. Системы POC представляют собой платформы, которые включают устройства и приложения для сбора, обработки и визуализации данных. Используя большие объемы данных, которые содержат личную информацию о здоровье и конфиденциальные медицинские данные, передаваемые через различные системы POC, внутренние аналитические платформы, рабочие станции пользователей и смартфоны, становится очевидным, что существует множество угроз, которые вызывают утечки данных или инциденты нарушения, которые наносят вред жизни пациента. Тестирование в месте оказания помощи уязвимо для утечек данных, которые сдерживают рост рынка
- Проблемы безопасности данных и конфиденциальности являются основными проблемами для рынка тестирования в точках оказания медицинской помощи (POCT) GSA и Бенилюкса. По мере того, как все больше устройств подключаются к цифровым платформам, увеличивается риск несанкционированного доступа и утечки данных, особенно в отношении конфиденциальной информации пациентов. Соблюдение таких норм, как HIPAA и GDPR, усложняет работу производителей и поставщиков медицинских услуг. Отсутствие надежной защиты в некоторых устройствах и опасения пациентов по поводу конфиденциальности могут замедлить внедрение, особенно в регионах с ограниченным доверием к цифровым медицинским решениям. Разработка безопасных систем, соответствующих требованиям конфиденциальности, имеет важное значение для преодоления этих барьеров.
Масштаб рынка тестирования в местах оказания медицинской помощи (POCT)
Рынок сегментирован по области применения, типу продукта, технологии, типу увеличения, конечному пользователю и каналу сбыта.
|
Сегментация |
Субсегментация |
|
По типу продукта |
|
|
По платформе |
|
|
По применению |
|
|
По рецепту
|
|
|
Конечным пользователем |
|
|
По каналу распространения
|
|
Ожидается, что в 2025 году продукция для мониторинга уровня глюкозы будет доминировать на рынке, занимая наибольшую долю в сегменте типов продукции.
Ожидается, что сегмент продукции для мониторинга уровня глюкозы будет доминировать на рынке тестирования в местах оказания медицинской помощи (poct) с наибольшей долей в 34,08% в 2025 году из-за высокой распространенности диабета и частой необходимости удобного и немедленного тестирования уровня глюкозы в крови.
Ожидается, что наибольшая доля в сегменте платформ в прогнозируемый период будет приходиться на анализы латерального потока/иммунохроматографические тесты.
Ожидается, что в 2025 году сегмент иммунохроматографических тестов с латеральным потоком будет доминировать на рынке, занимая наибольшую долю рынка в 37,28% благодаря своей простоте, быстроте результатов, экономической эффективности и пригодности для широкого спектра диагностических применений за пределами традиционных лабораторных условий.
Региональный анализ рынка тестирования в точках оказания медицинской помощи (POCT)
«Германия занимает самую большую долю на рынке тестирования в пунктах оказания медицинской помощи (POCT)»
- Германия доминирует на рынке тестирования в местах оказания медицинской помощи (POCT) с долей 82,06% в регионе GSA, что обусловлено высокоразвитой инфраструктурой здравоохранения, широким внедрением инновационных диагностических технологий и сильным акцентом на профилактическую и персонализированную медицину.
- Страна занимает значительную долю благодаря растущему спросу на быстрые и точные диагностические решения, особенно при лечении хронических заболеваний, таких как диабет, сердечно-сосудистые заболевания и респираторные инфекции.
- Поддерживающие системы возмещения расходов, поддерживаемые государством инициативы в области цифрового здравоохранения и надежные инвестиции в НИОКР в сфере здравоохранения еще больше стимулируют рост рынка
- Кроме того, все более широкое внедрение POCT в отделениях неотложной помощи, учреждениях первичной медико-санитарной помощи и домашнем медицинском обслуживании в сочетании с акцентом на снижение нагрузки на больницы стимулирует расширение рынка по всей Германии.
«По прогнозам, в Нидерландах будет зарегистрирован самый высокий среднегодовой темп роста на рынке тестирования по месту оказания медицинской помощи (POCT)»
- Ожидается, что в Нидерландах будет наблюдаться устойчивый рост рынка тестирования в местах оказания медицинской помощи (POCT) с долей в 54,40% , что обусловлено акцентом на цифровое здравоохранение, раннее выявление заболеваний и модели оказания помощи, ориентированные на пациента.
- Старение населения страны и рост распространенности хронических заболеваний, таких как диабет, сердечно-сосудистые заболевания и респираторные расстройства, обусловливают потребность в быстрых и децентрализованных диагностических решениях.
- Благодаря хорошо развитой системе первичной медико-санитарной помощи и прогрессивной политике в области здравоохранения Нидерланды остаются ключевым государством, внедряющим технологии POCT, особенно в общественных медицинских учреждениях, клиниках врачей общей практики (ВОП) и службах ухода на дому.
- Растущая интеграция устройств POCT с электронными медицинскими картами (ЭМК), поддержка со стороны медицинских страховщиков в отношении диагностических инноваций и активное участие в общеевропейских инициативах в области медицинских технологий еще больше укрепляют перспективы рынка.
- Сотрудничество между академическими учреждениями, технологическими стартапами и диагностическими компаниями ускоряет инновации и локальную разработку передовых, удобных для пользователя решений POCT, адаптированных к голландской системе здравоохранения.
Доля рынка тестирования в местах оказания медицинской помощи (POCT)
Конкурентная среда рынка содержит сведения о конкурентах. Включены сведения о компании, ее финансах, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, глобальном присутствии, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта, доминировании приложений. Приведенные выше данные касаются только фокуса компаний на рынке.
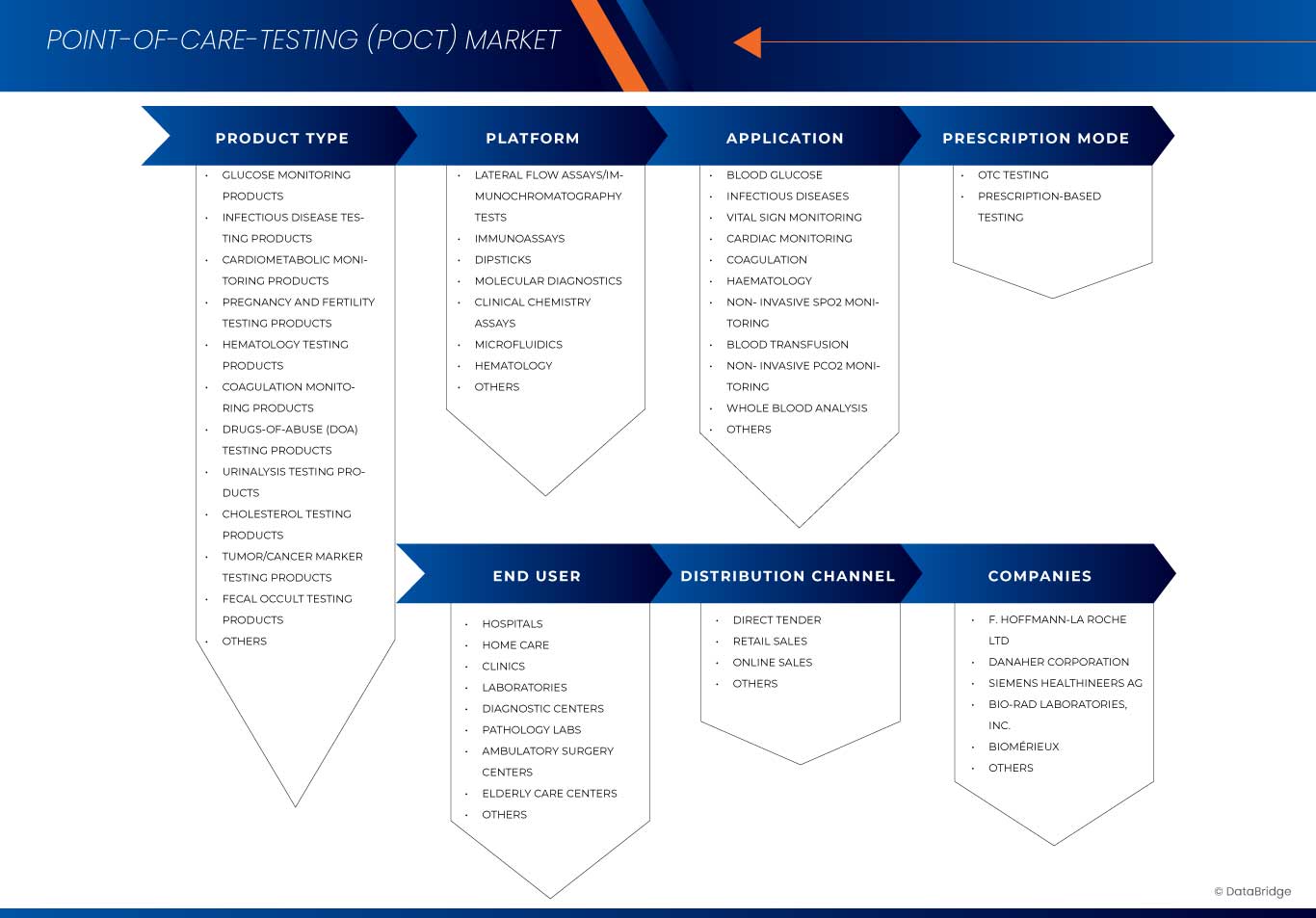
Основными лидерами рынка, работающими на рынке, являются:
- F. Hoffmann-La Roche Ltd (Швейцария)
- Корпорация Danaher (США)
- Siemens Healthineers AG (Германия)
- Bio-Rad Laboratories, Inc. (США)
- БИОМЕРЬЕ (Франция)
- БД (США)
- Корпорация QuidelOrtho (США)
- Hologic, Inc. (США)
- Thermo Fisher Scientific Inc. (США)
- Эбботт (США)
- Верфен (Испания)
- QIAGEN (Германия)
- Корпорация Sysmex (Япония)
- EKF Diagnostics Holdings plc. (Великобритания)
- Sekisui Diagnostics (США)
- Meridian Bioscience (США)
- binx health, inc. (США)
- Trinity Biotech (Ирландия)
- Sienco, Inc. (США)
- ACCUBIOTECH CO., LTD. (Китай)
Последние разработки на рынке тестирования в пунктах оказания медицинской помощи (POCT) в странах GSA и Бенилюкса
- В декабре 2023 года Roche приобрела отдельные части инновационной технологии Point of Care компании LumiraDx, расширив свой диагностический портфель. Это приобретение позволяет проводить более быстрое и доступное тестирование в различных областях заболеваний. Интеграция платформы LumiraDx улучшит доступ пациентов к своевременным и точным результатам диагностики в децентрализованных медицинских учреждениях по всему миру
- В январе 2025 года корпорация Danaher сформировала инвестиционное партнерство с Innovaccer Inc., компанией, занимающейся ИИ в здравоохранении. Целью этого сотрудничества является ускорение внедрения точной диагностики и ценностно-ориентированного ухода путем предоставления поставщикам медицинских услуг унифицированных данных пациентов и расширенной аналитики, что позволит улучшить результаты лечения пациентов за счет персонализированных своевременных вмешательств.
- В октябре 2024 года компания bioMérieux призвала к многосекторальному сотрудничеству для продвижения глобальной повестки дня по устойчивости к противомикробным препаратам (УПП). Компания подчеркивает настоятельную необходимость объединения усилий здравоохранения, правительства и промышленности для решения проблемы УПП, продвижения инновационных диагностических решений и ответственного использования противомикробных препаратов для борьбы с этой растущей глобальной угрозой здоровью
- В марте 2024 года компания Bio-Rad выпустила решение XP-Design Assay Salmonella Serotyping Solution для быстрого и точного тестирования безопасности пищевых продуктов. Решение XP-Design Assay Salmonella Serotyping Solution обеспечило гибкий подход к идентификации серотипов Salmonella после первоначального скрининга. Готовый к использованию раствор содержит олигонуклеотиды (праймеры и зонды), специфичные для целевого серотипа, что обеспечивает высокую специфичность и снижает риск перекрестной реакции, обеспечивая надежные результаты.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Содержание
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 YEARS CONSIDERED FOR THE STUDY
2.3 CURRENCY AND PRICING
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 PRODUCT TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 MICRO AND MACRO ECONOMIC FACTORS
4.4 PENETRATION AND GROWTH PROSPECT MAPPING
4.5 KEY PRICING STRATEGIES
4.6 COST ANALYSIS BREAKDOWN
4.7 OPPORTUNITY MAP ANALYSIS
4.8 VALUE CHAIN ANALYSIS
4.9 HEALTHCARE ECONOMY
4.1 TARIFFS
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 GLOBAL VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 16.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 CUSTOMIZATION
5.1 LEADING DIGITAL HEALTH PLATFORMS IN EACH COUNTRY –
5.2 POCT DATA INTEGRATION USE CASES –
5.3 INFORMATICS PLATFORMS & TELEMEDICINE INTEGRATION:
6 REGULATORY
6.1 REGULATORY GSA
6.2 REGULATORY BENELUX
7 MARKET OVERVIEW
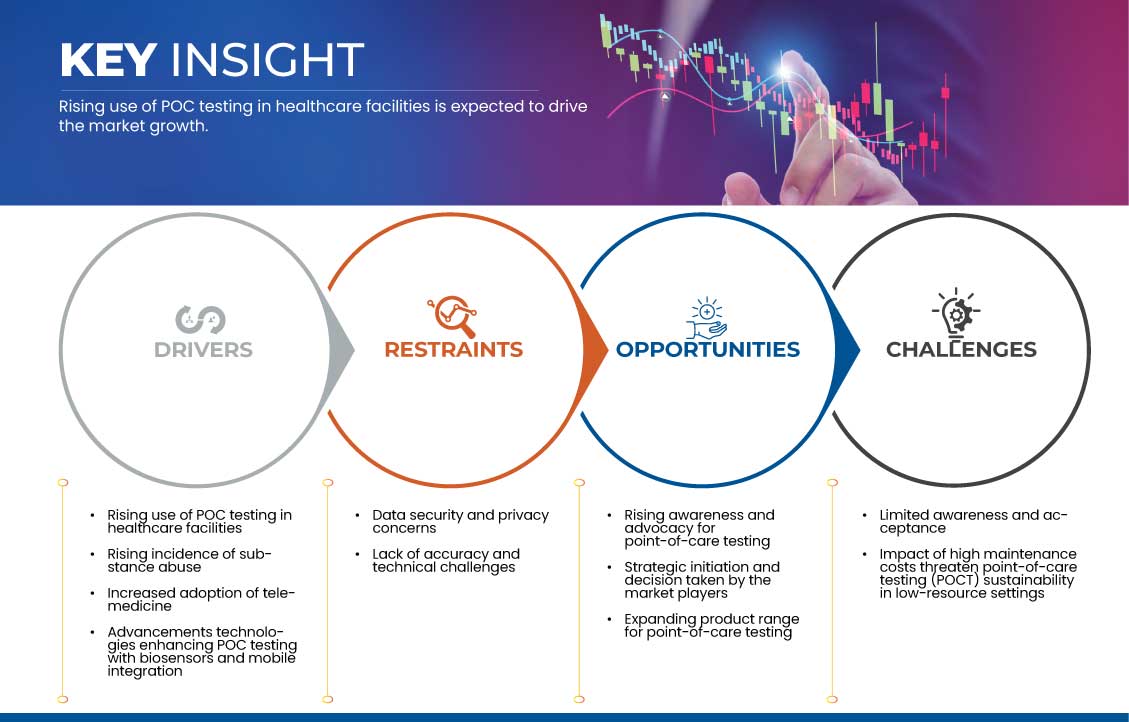
7.1 DRIVERS
7.1.1 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES
7.1.2 RISING INCIDENCE OF SUBSTANCE ABUSE
7.1.3 INCREASED ADOPTION OF TELEMEDICINE
7.1.4 ADVANCEMENTS TECHNOLOGIES ENHANCING POC TESTING WITH BIOSENSORS AND MOBILE INTEGRATION
7.2 RESTRAINTS
7.2.1 DATA SECURITY AND PRIVACY CONCERNS
7.2.2 ACCURACY LIMITATIONS AND TECHNICAL BARRIERS
7.3 OPPORTUNITIES
7.3.1 RISING AWARENESS AND ADVOCACY FOR POINT-OF-CARE TESTING
7.3.2 STRATEGIC INITIATION AND DECISION TAKEN BY THE MARKET PLAYERS
7.3.3 EXPANDING PRODUCT RANGE FOR POINT-OF-CARE TESTING
7.4 CHALLENGES
7.4.1 LIMITED AWARENESS AND ACCEPTANCE OF POINT-OF-CARE (POC) TESTING
7.4.2 IMPACT OF HIGH MAINTENANCE COSTS THREATEN POINT-OF-CARE TESTING (POCT) SUSTAINABILITY IN LOW-RESOURCE SETTINGS
8 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 GLUCOSE MONITORING PRODUCTS
8.2.1 STRIPS
8.2.2 METERS
8.2.3 LANCETS AND LANCING DEVICES
8.3 INFECTIOUS DISEASE TESTING PRODUCTS
8.3.1 COVID-19
8.3.2 HIV TESTING PRODUCTS
8.3.2.1 TESTING REAGENTS
8.3.2.2 TESTING EQUIPMENT
8.3.3 RESPIRATORY INFECTION TESTING PRODUCTS
8.3.4 SEXUALLY TRANSMITTED DISEASES (STD) TESTING
8.3.4.1 NAAT-BASED SYSTEMS
8.3.4.2 NON–NAAT-BASED SYSTEMS
8.3.5 HEPATITIS C TESTING PRODUCTS
8.3.5.1 HCV ANTIBODY TESTS
8.3.5.2 HCV VIRAL LOAD TESTS
8.3.6 INFLUENZA TESTING PRODUCTS
8.3.6.1 TRADITIONAL DIAGNOSTIC TEST
8.3.6.1.1 RAPID INFLUENZA DIAGNOSTIC TEST (RIDT)
8.3.6.1.2 DIRECT FLUORESCENT ANTIBODY TEST (DFAT)
8.3.6.1.3 VIRAL CULTURE
8.3.6.1.4 SEROLOGICAL ASSAY
8.3.6.2 MOLECULAR DIAGNOSTIC ASSAY
8.3.6.2.1 RT-PCR
8.3.6.2.2 LOOP-MEDIATED ISOTHERMAL AMPLIFICATION-BASED ASSAY (LAMP)
8.3.6.2.3 NUCLEIC ACID SEQUENCE-BASED AMPLIFICATION TEST (NASBAT)
8.3.6.2.4 SIMPLE AMPLIFICATION-BASED ASSAY (SAMBA)
8.3.7 HEALTHCARE ASSOCIATED INFECTION (HAI) TESTING
8.3.8 TROPICAL DISEASE TESTING PRODUCTS
8.3.9 OTHER INFECTIOUS DISEASE TESTING PRODUCTS
8.4 CARDIOMETABOLIC MONITORING PRODUCTS
8.4.1 CARDIAC MARKER TESTING PRODUCTS
8.4.1.1 HSTNL
8.4.1.2 BNP
8.4.1.3 D-DIMER
8.4.1.4 CK-MB
8.4.1.5 MYOGLOBIN
8.4.2 BLOOD GAS/ELECTROLYTE TESTING PRODUCTS
8.4.3 BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES
8.4.3.1 CARTRIDGES
8.4.3.2 REAGENTS
8.4.4 BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS
8.4.4.1 PORTABLE
8.4.4.1.1 COMBINED ANALYZERS
8.4.4.1.2 BLOOD GAS ANALYZERS
8.4.4.1.3 ELECTROLYTE ANALYZERS
8.4.4.2 BENCHTOP
8.4.4.2.1 COMBINED ANALYZERS
8.4.4.2.2 BLOOD GAS ANALYZERS
8.4.4.2.3 ELECTROLYTE ANALYZERS
8.4.5 HBA1C TESTING PRODUCTS
8.4.5.1 HBA1C TESTING INSTRUMENTS
8.4.5.2 HBA1C TESTING CONSUMABLES
8.4.6 POC ANALYZER
8.4.7 ECG DEVICE
8.4.7.1 RESTING ECG DEVICES
8.4.7.2 STRESS ECG DEVICES
8.4.7.3 HOLTER MONITORS
8.5 PREGNANCY AND FERTILITY TESTING PRODUCTS
8.5.1 PREGNANCY TESTING PRODUCTS
8.5.1.1 STRIPS/ DIP STICKS AND CARDS
8.5.1.2 MID STREAM DEVICES
8.5.1.3 CASSETTES
8.5.1.4 DIGITAL DEVICES
8.5.1.5 LINE-INDICATOR DEVICES
8.5.2 FERTILITY TESTING PRODUCTS
8.5.2.1 LUTEINIZING HORMONE (LH) URINE TEST
8.5.2.2 FSH TEST
8.5.2.3 OTHERS
8.6 HAEMATOLOGY TESTING PRODUCTS
8.7 COAGULATION MONITORING PRODUCTS
8.7.1 ANTICOAGULATION MONITORING DEVICES
8.7.1.1 PROTHROMBIN TIME/INTERNATIONAL NORMALIZED RATIO (PT-INR) TESTING DEVICES
8.7.1.2 ACTIVATED CLOTTING TIME (ACT)
8.7.1.3 ACTIVATED PARTIAL THROMBOPLASTIN TIME (APPT)
8.7.2 PLATELET FUNCTION MONITORING DEVICES
8.7.3 VISCOELASTIC COAGULATION MONITORING DEVICES
8.7.3.1 ROTATIONAL THROMBOELASTOMETRY (ROTEM)
8.7.3.2 THROMBOELASTOGRAPHY (TEG)
8.8 DRUG-OF-ABUSE (DOA) TESTING PRODUCTS
8.8.1 DOA ANALYSERS
8.8.1.1 IMMUNOASSAYS
8.8.1.2 CHROMATOGRAPHIC DEVICES
8.8.1.3 BREATH ANALYSERS
8.8.2 RAPID TESTING DEVICES
8.8.2.1 URINE TESTING DEVICES
8.8.2.2 ORAL FLUID TESTING DEVICES
8.8.2.3 OTHERS
8.9 URINALYSIS TESTING PRODUCTS
8.9.1 POC URINE STRIP SELF-TESTING
8.9.2 POC URINE TEST STRIP PROFESSIONAL TESTING
8.1 CHOLESTEROL TESTING PRODUCTS
8.10.1 TESTING KITS
8.10.2 INSTRUMENTS
8.10.2.1 TABLE-TOP ANALYZERS
8.10.2.2 HAND-HELD ANALYZERS
8.11 TUMOR/CANCER MARKER TESTING PRODUCTS
8.12 FECAL OCCULT TESTING PRODUCTS
8.12.1 GUAIAC FOB STOOL TEST
8.12.2 LATERAL FLOW IMMUNO-FOB TEST
8.12.3 IMMUNO-FOB AGGLUTINATION TEST
8.12.4 IMMUNO-FOB ELISA TEST
8.13 OTHERS
9 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY PRESCRIPTION MODE
9.1 OVERVIEW
9.2 OTC TESTING
9.3 PRESCRIPTION-BASED TESTING
10 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY PLATFORM
10.1 OVERVIEW
10.2 LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS
10.3 IMMUNOASSAYS
10.4 DIPSTICKS
10.5 MOLECULAR DIAGNOSTICS
10.6 CLINICAL CHEMISTRY ASSAYS
10.7 MICROFLUIDICS
10.8 HEMATOLOGY
10.9 OTHERS
11 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 BLOOD GLUCOSE
11.3 INFECTIOUS DISEASES
11.3.1 COVID-19 TESTING
11.3.2 HIV TESTING
11.3.3 HEPATITIS C TESTING
11.3.4 INFLUENZA TESTING
11.3.5 TUBERCULOSIS TESTING
11.3.6 OTHERS
11.4 VITAL SIGN MONITORING
11.5 CARDIAC MONITORING
11.6 COAGULATION
11.7 HAEMATOLOGY
11.8 NON- INVASIVE SPO2 MONITORING
11.9 BLOOD TRANSFUSION
11.1 NON- INVASIVE PCO2 MONITORING
11.11 WHOLE BLOOD ANALYSIS
11.12 OTHERS
12 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 PRIVATE
12.2.1.1 TIER 1
12.2.1.2 TIER 2
12.2.1.3 TIER 3
12.2.2 PUBLIC
12.3 HOME CARE
12.4 CLINICS
12.5 LABORATORIES
12.6 DIAGNOSTIC CENTERS
12.7 PATHOLOGY LABS
12.8 AMBULATORY SURGERY CENTERS
12.9 ELDERLY CARE CENTERS
12.1 OTHERS
13 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
13.4 ONLINE SALES
13.5 OTHERS
14 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY PRODUCT TYPE
14.1 OVERVIEW
14.2 GLUCOSE MONITORING PRODUCTS
14.2.1 STRIPS
14.2.2 METERS
14.2.3 LANCETS AND LANCING DEVICES
14.3 INFECTIOUS DISEASE TESTING PRODUCTS
14.3.1 COVID-19
14.3.2 HIV TESTING PRODUCTS
14.3.2.1 TESTING REAGENTS
14.3.2.2 TESTING EQUIPMENT
14.3.3 RESPIRATORY INFECTION TESTING PRODUCTS
14.3.4 SEXUALLY TRANSMITTED DISEASES (STD) TESTING
14.3.5 NAAT-BASED SYSTEMS
14.3.6 NON–NAAT-BASED SYSTEMS
14.3.7 HEPATITIS C TESTING PRODUCTS
14.3.7.1 HCV ANTIBODY TESTS
14.3.7.2 HCV VIRAL LOAD TESTS
14.3.8 INFLUENZA TESTING PRODUCTS
14.3.8.1 TRADITIONAL DIAGNOSTIC TEST
14.3.8.1.1 RAPID INFLUENZA DIAGNOSTIC TEST (RIDT)
14.3.8.1.2 DIRECT FLUORESCENT ANTIBODY TEST (DFAT)
14.3.8.1.3 VIRAL CULTURE
14.3.8.1.4 SEROLOGICAL ASSAY
14.3.8.2 MOLECULAR DIAGNOSTIC ASSAY
14.3.8.2.1 RT-PCR
14.3.8.2.2 LOOP-MEDIATED ISOTHERMAL AMPLIFICATION-BASED ASSAY (LAMP)
14.3.8.2.3 NUCLEIC ACID SEQUENCE-BASED AMPLIFICATION TEST (NASBAT)
14.3.8.2.4 SIMPLE AMPLIFICATION-BASED ASSAY (SAMBA)
14.3.9 HEALTHCARE ASSOCIATED INFECTION (HAI) TESTING
14.3.10 TROPICAL DISEASE TESTING PRODUCTS
14.3.11 OTHER INFECTIOUS DISEASE TESTING PRODUCTS
14.4 CARDIOMETABOLIC MONITORING PRODUCTS
14.4.1 CARDIAC MARKER TESTING PRODUCTS
14.4.1.1 HSTNL
14.4.1.2 BNP
14.4.1.3 D-DIMER
14.4.1.4 CK-MB
14.4.1.5 MYOGLOBIN
14.4.2 BLOOD GAS/ELECTROLYTE TESTING PRODUCTS
14.4.3 BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES
14.4.3.1 CARTRIDGES
14.4.3.2 REAGENTS
14.4.4 BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS
14.4.5 PORTABLE
14.4.5.1 COMBINED ANALYZERS
14.4.5.2 BLOOD GAS ANALYZERS
14.4.5.3 ELECTROLYTE ANALYZERS
14.4.6 BENCHTOP
14.4.6.1 COMBINED ANALYZERS
14.4.6.2 BLOOD GAS ANALYZERS
14.4.6.3 ELECTROLYTE ANALYZERS
14.4.7 HBA1C TESTING PRODUCTS
14.4.7.1 HBA1C TESTING INSTRUMENTS
14.4.7.2 HBA1C TESTING CONSUMABLES
14.4.8 POC ANALYZER
14.4.9 ECG DEVICE
14.4.9.1 RESTING ECG DEVICES
14.4.9.2 STRESS ECG DEVICES
14.4.9.3 HOLTER MONITORS
14.5 PREGNANCY AND FERTILITY TESTING PRODUCTS
14.5.1 PREGNANCY TESTING PRODUCTS
14.5.1.1 STRIPS/ DIP STICKS AND CARDS
14.5.1.2 MID STREAM DEVICES
14.5.1.3 CASSETTES
14.5.1.4 DIGITAL DEVICES
14.5.1.5 LINE-INDICATOR DEVICES
14.5.2 FERTILITY TESTING PRODUCTS
14.5.2.1 LUTEINIZING HORMONE (LH) URINE TEST
14.5.2.2 FSH TEST
14.5.2.3 OTHERS
14.6 HAEMATOLOGY TESTING PRODUCTS
14.7 COAGULATION MONITORING PRODUCTS
14.8 ANTICOAGULATION MONITORING DEVICES
14.8.1 PROTHROMBIN TIME/INTERNATIONAL NORMALIZED RATIO (PT-INR) TESTING DEVICES
14.8.2 ACTIVATED CLOTTING TIME (ACT)
14.8.3 ACTIVATED PARTIAL THROMBOPLASTIN TIME (APPT)
14.8.4 PLATELET FUNCTION MONITORING DEVICES
14.8.5 VISCOELASTIC COAGULATION MONITORING DEVICES
14.8.5.1 ROTATIONAL THROMBOELASTOMETRY (ROTEM)
14.8.5.2 THROMBOELASTOGRAPHY (TEG)
14.9 DRUG-OF-ABUSE (DOA) TESTING PRODUCTS
14.9.1 DOA ANALYSERS
14.9.1.1 IMMUNOASSAYS
14.9.1.2 CHROMATOGRAPHIC DEVICES
14.9.1.3 BREATH ANALYSERS
14.9.2 RAPID TESTING DEVICES
14.9.2.1 URINE TESTING DEVICES
14.9.2.2 ORAL FLUID TESTING DEVICES
14.9.3 OTHERS
14.1 URINALYSIS TESTING PRODUCTS
14.10.1 POC URINE STRIP SELF-TESTING
14.10.2 POC URINE TEST STRIP PROFESSIONAL TESTING
14.11 CHOLESTEROL TESTING PRODUCTS
14.11.1 TESTING KITS
14.11.2 INSTRUMENTS
14.11.2.1 TABLE-TOP ANALYZERS
14.11.2.2 HAND-HELD ANALYZERS
14.12 TUMOR/CANCER MARKER TESTING PRODUCTS
14.13 FECAL OCCULT TESTING PRODUCTS
14.13.1 GUAIAC FOB STOOL TEST
14.13.2 LATERAL FLOW IMMUNO-FOB TEST
14.13.3 IMMUNO-FOB AGGLUTINATION TEST
14.13.4 IMMUNO-FOB ELISA TEST
14.14 OTHERS
15 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY PLATFORM
15.1 OVERVIEW
15.2 LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS
15.3 IMMUNOASSAYS
15.4 DIPSTICKS
15.5 MOLECULAR DIAGNOSTICS
15.6 CLINICAL CHEMISTRY ASSAYS
15.7 MICROFLUIDICS
15.8 HEMATOLOGY
15.9 OTHERS
16 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY PRESCRIPTION MODE
16.1 OVERVIEW
16.2 OTC TESTING
16.3 PRESCRIPTION-BASED TESTING
17 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION
17.1 OVERVIEW
17.2 BLOOD GLUCOSE
17.3 INFECTIOUS DISEASES
17.3.1.1 COVID-19 TESTING
17.3.1.2 HIV TESTING
17.3.1.3 HEPATITIS C TESTING
17.3.1.4 INFLUENZA TESTING
17.3.1.5 TUBERCULOSIS TESTING
17.3.1.6 OTHERS
17.4 VITAL SIGN MONITORING
17.5 CARDIAC MONITORING
17.6 COAGULATION
17.7 HAEMATOLOGY
17.8 NON- INVASIVE SPO2 MONITORING
17.9 BLOOD TRANSFUSION
17.1 NON- INVASIVE PCO2 MONITORING
17.11 WHOLE BLOOD ANALYSIS
17.12 OTHERS
18 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY END USER
18.1 OVERVIEW
18.2 HOSPITALS
18.2.1 PRIVATE
18.2.1.1 TIER 1
18.2.1.2 TIER 2
18.2.1.3 TIER 3
18.2.2 PUBLIC
18.3 HOME CARE
18.4 CLINICS
18.5 LABORATORIES
18.6 DIAGNOSTIC CENTERS
18.7 PATHOLOGY LABS
18.8 AMBULATORY SURGERY CENTERS
18.9 ELDERLY CARE CENTERS
18.1 OTHERS
19 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL
19.1 OVERVIEW
19.2 DIRECT TENDER
19.3 RETAIL SALES
19.4 ONLINE SALES
19.5 OTHERS
20 GSA & BENELUX
20.1 GERMANY
20.2 NETHERLANDS
20.3 SWITZERLAND
20.4 BELGIUM
20.5 AUSTRIA
20.6 LUXEMBOURG
21 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY LANDSCAPE
21.1 COMPANY SHARE ANALYSIS: GSA AND BENELUX
22 SWOT ANALYSIS
23 COMPANY PROFILE
23.1 F. HOFFMANN-LA ROCHE LTD
23.1.1 COMPANY SNAPSHOT
23.1.2 REVENUE ANALYSIS
23.1.3 PRODUCT PORTFOLIO
23.1.4 RECENT DEVELOPMENT
23.2 DANAHER CORPORATION
23.2.1 COMPANY SNAPSHOT
23.2.2 REVENUE ANALYSIS
23.2.3 PRODUCT PORTFOLIO
23.2.4 RECENT DEVELOPMENT
23.3 SIEMENS HEALTHINEERS AG
23.3.1 COMPANY SNAPSHOT
23.3.2 REVENUE ANALYSIS
23.3.3 PRODUCT PORTFOLIO
23.3.4 RECENT DEVELOPMENT
23.4 BIO- RAD LABORATORIES, INC.
23.4.1 COMPANY SNAPSHOT
23.4.2 REVENUE ANALYSIS
23.4.3 PRODUCT PORTFOLIO
23.4.4 RECENT DEVELOPMENT
23.5 BIOMERIEUX
23.5.1 COMPANY SNAPSHOT
23.5.2 REVENUE ANALYSIS
23.5.3 PRODUCT PORTFOLIO
23.5.4 RECENT DEVELOPMENT
23.6 ACCUBIOTECH CO., LTD.
23.6.1 COMPANY SNAPSHOT
23.6.2 PRODUCT PORTFOLIO
23.6.3 RECENT DEVELOPMENT
23.7 ABBOTT
23.7.1 COMPANY SNAPSHOT
23.7.2 REVENUE ANALYSIS
23.7.3 PRODUCT PORTFOLIO
23.7.4 RECENT DEVELOPMENT/NEWS
23.8 BINX HEALTH
23.8.1 COMPANY SNAPSHOT
23.8.2 SOLUTION PORTFOLIO
23.8.3 RECENT DEVELOPMENT
23.9 BD
23.9.1 COMPANY SNAPSHOT
23.9.2 REVENUE ANALYSIS
23.9.3 PRODUCT PORTFOLIO
23.9.4 RECENT DEVELOPMENT/NEWS
23.1 EKF DIAGNOSTICS HOLDINGS PLC
23.10.1 COMPANY SNAPSHOT
23.10.2 REVENUE ANALYSIS
23.10.3 PRODUCT PORTFOLIO
23.10.4 RECENT DEVELOPMENT
23.11 HOLOGIC, INC
23.11.1 COMPANY SNAPSHOT
23.11.2 REVENUE ANALYSIS
23.11.3 PRODUCT PORTFOLIO
23.11.4 RECENT DEVELOPMENT
23.12 MERIDIAN BIOSCIENCE, INC.
23.12.1 COMPANY SNAPSHOT
23.12.2 PRODUCT PORTFOLIO
23.12.3 RECENT DEVELOPMENT/NEWS
23.13 QIAGEN
23.13.1 COMPANY SNAPSHOT
23.13.2 REVENUE ANALYSIS
23.13.3 PRODUCT PORTFOLIO
23.13.4 RECENT DEVELOPMENT
23.14 QUIDELORTHO CORPORATION
23.14.1 COMPANY SNAPSHOT
23.14.2 REVENUE ANALYSIS
23.14.3 PRODUCT PORTFOLIO
23.14.4 RECENT DEVELOPMENT
23.15 SEKISUI DIAGNOSTICS
23.15.1 COMPANY SNAPSHOT
23.15.2 PRODUCT PORTFOLIO
23.15.3 RECENT DEVELOPMENT
23.16 SYSMEX CORPORATION
23.16.1 COMPANY SNAPSHOT
23.16.2 REVENUE ANALYSIS
23.16.3 PRODUCT PORTFOLIO
23.16.4 RECENT DEVELOPMENT
23.17 SIENCO, INC.
23.17.1 COMPANY SNAPSHOT
23.17.2 PRODUCT PORTFOLIO
23.17.3 RECENT DEVELOPMENT
23.18 TRINITY BIOTECH
23.18.1 COMPANY SNAPSHOT
23.18.2 REVENUE ANALYSIS
23.18.3 PRODUCT PORTFOLIO
23.18.4 RECENT DEVELOPMENT
23.19 THERMO FISHER SCIENTIFIC INC.
23.19.1 COMPANY SNAPSHOT
23.19.2 REVENUE ANALYSIS
23.19.3 PRODUCT PORTFOLIO
23.19.4 RECENT DEVELOPMENT
23.2 WERFEN
23.20.1 COMPANY SNAPSHOT
23.20.2 PRODUCT PORTFOLIO
23.20.3 RECENT DEVELOPMENT
24 QUESTIONNAIRE
Список таблиц
TABLE 1 HOME-USE POCT DEVICES WITH TELEMEDICINE INTEGRATION
TABLE 2 COMPANIES AND PRODUCTS WITH TELEMEDICINE INTEGRATION
TABLE 3 A TECHNOLOGY ROADMAP OUTLINING THE FUTURE OF CONNECTED POCT DEVICES
TABLE 4 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 6 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 7 GSA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 GSA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 9 GSA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 10 GSA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 11 GSA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 12 GSA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 13 GSA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 GSA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 15 GSA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 16 GSA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 GSA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 18 GSA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 19 GSA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 20 GSA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 21 GSA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 22 GSA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 23 GSA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 24 GSA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 25 GSA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 26 GSA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 27 GSA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 28 GSA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 29 GSA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 GSA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 31 GSA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 32 GSA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 GSA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 34 GSA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 35 GSA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 GSA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 GSA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 38 GSA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 39 GSA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 40 GSA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 GSA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 42 GSA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 43 GSA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 GSA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 45 GSA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 46 GSA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 47 GSA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 48 GSA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 49 GSA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 GSA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 51 GSA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 52 GSA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 GSA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 GSA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 55 GSA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 56 GSA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 GSA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 58 GSA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 59 GSA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 GSA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 GSA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 62 GSA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 63 GSA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 64 GSA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 GSA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 66 GSA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNIT)
TABLE 67 GSA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 68 GSA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 GSA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 70 GSA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 71 GSA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 GSA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 73 GSA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 74 GSA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 GSA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 76 GSA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 77 GSA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 GSA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 79 GSA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 80 GSA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 GSA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 82 GSA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 83 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 84 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 85 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 86 GSA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 88 GSA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 GSA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 90 GSA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 91 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 92 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 94 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 95 BENELUX GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 96 BENELUX GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 97 BENELUX GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 98 BENELUX INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 BENELUX INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 100 BENELUX INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 101 BENELUX HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 BENELUX HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 103 BENELUX HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 104 BENELUX SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 BENELUX SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 106 BENELUX SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 107 BENELUX HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 BENELUX HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 109 BENELUX HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 110 BENELUX INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 111 BENELUX TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 BENELUX TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 113 BENELUX TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 114 BENELUX MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 BENELUX MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 116 BENELUX MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 117 BENELUX CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 BENELUX POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 119 BENELUX POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 120 BENELUX CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 BENELUX CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 122 BENELUX CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 123 BENELUX BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 124 BENELUX BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 BENELUX BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 126 BENELUX BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 127 BENELUX BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 128 BENELUX PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 BENELUX PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 130 BENELUX PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 131 BENELUX BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 BENELUX BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 133 BENELUX BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 134 BENELUX HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 BENELUX HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 136 BENELUX HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 137 BENELUX ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 BENELUX ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 139 BENELUX ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 140 BENELUX PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 BENELUX PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 142 BENELUX PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 143 BENELUX PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 144 BENELUX FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 BENELUX FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 146 BENELUX FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 147 BENELUX COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 BENELUX ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 149 BENELUX ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 150 BENELUX ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 151 BENELUX VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 152 BENELUX DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 153 BENELUX DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 BENELUX DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNIT)
TABLE 155 BENELUX DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 156 BENELUX RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 BENELUX RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 158 BENELUX RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 159 BENELUX URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 BENELUX URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 161 BENELUX URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 162 BENELUX CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 BENELUX CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 164 BENELUX CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 165 BENELUX INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 166 BENELUX INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 167 BENELUX INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 168 BENELUX FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 BENELUX FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 170 BENELUX FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 171 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 172 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 173 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 174 BENELUX INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 176 BENELUX HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 BENELUX PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 178 BENELUX PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 179 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 180 GSA & BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 181 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 182 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 183 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 185 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 186 GERMANY GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 187 GERMANY GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 188 GERMANY GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 189 GERMANY INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 GERMANY INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 191 GERMANY INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 192 GERMANY HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 GERMANY HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 194 GERMANY HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 195 GERMANY SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 GERMANY SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 197 GERMANY SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 198 GERMANY HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 199 GERMANY HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 200 GERMANY HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 201 GERMANY INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 GERMANY TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 GERMANY TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 204 GERMANY TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 205 GERMANY MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 206 GERMANY MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 207 GERMANY MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 208 GERMANY CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 GERMANY POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 210 GERMANY POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 211 GERMANY CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 GERMANY CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 213 GERMANY CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 214 GERMANY BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 GERMANY BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 GERMANY BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 217 GERMANY BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 218 GERMANY BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 219 GERMANY PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 GERMANY PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 221 GERMANY PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 222 GERMANY BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 GERMANY BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 224 GERMANY BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 225 GERMANY HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 GERMANY HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 227 GERMANY HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 228 GERMANY ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 229 GERMANY ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 230 GERMANY ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 231 GERMANY PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 GERMANY PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 GERMANY PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 234 GERMANY PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 235 GERMANY FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 GERMANY FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 237 GERMANY FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 238 GERMANY COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 GERMANY ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 GERMANY ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 241 GERMANY ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 242 GERMANY VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 243 GERMANY DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 244 GERMANY DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 GERMANY DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 246 GERMANY DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 247 GERMANY RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 248 GERMANY RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 249 GERMANY RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 250 GERMANY URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 GERMANY URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 252 GERMANY URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 253 GERMANY CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 GERMANY CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 255 GERMANY CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 256 GERMANY INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 257 GERMANY INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 258 GERMANY INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 259 GERMANY FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 GERMANY FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 261 GERMANY FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 262 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 263 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 264 GERMANY INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 266 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 267 GERMANY HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 GERMANY PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 269 GERMANY PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 270 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 271 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 273 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 274 NETHERLANDS GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 275 NETHERLANDS GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 276 NETHERLANDS GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 277 NETHERLANDS INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 NETHERLANDS INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 279 NETHERLANDS INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 280 NETHERLANDS HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 NETHERLANDS HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 282 NETHERLANDS HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 283 NETHERLANDS SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 NETHERLANDS SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 285 NETHERLANDS SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 286 NETHERLANDS HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 NETHERLANDS HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 288 NETHERLANDS HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 289 NETHERLANDS INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 NETHERLANDS TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 NETHERLANDS TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 292 NETHERLANDS TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 293 NETHERLANDS MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 NETHERLANDS MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 295 NETHERLANDS MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 296 NETHERLANDS CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 NETHERLANDS POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 298 NETHERLANDS POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 299 NETHERLANDS CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 NETHERLANDS CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 301 NETHERLANDS CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 302 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 305 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 306 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 NETHERLANDS PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 NETHERLANDS PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 309 NETHERLANDS PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 310 NETHERLANDS BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 NETHERLANDS BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 312 NETHERLANDS BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 313 NETHERLANDS HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 314 NETHERLANDS HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 315 NETHERLANDS HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 316 NETHERLANDS ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 NETHERLANDS ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 318 NETHERLANDS ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 319 NETHERLANDS PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 320 NETHERLANDS PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 NETHERLANDS PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 322 NETHERLANDS PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 323 NETHERLANDS FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 324 NETHERLANDS FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 325 NETHERLANDS FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 326 NETHERLANDS COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 NETHERLANDS ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 NETHERLANDS ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 329 NETHERLANDS ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 330 NETHERLANDS VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 331 NETHERLANDS DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 332 NETHERLANDS DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 333 NETHERLANDS DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 334 NETHERLANDS DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 335 NETHERLANDS RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 336 NETHERLANDS RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 337 NETHERLANDS RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 338 NETHERLANDS URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 339 NETHERLANDS URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 340 NETHERLANDS URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 341 NETHERLANDS CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 342 NETHERLANDS CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 343 NETHERLANDS CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 344 NETHERLANDS INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 345 NETHERLANDS INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 346 NETHERLANDS INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 347 NETHERLANDS FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 348 NETHERLANDS FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 349 NETHERLANDS FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 350 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 351 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 352 NETHERLANDS INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 353 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 354 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 355 NETHERLANDS HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 356 NETHERLANDS PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 357 NETHERLANDS PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 358 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 359 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 360 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 361 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 362 SWITZERLAND GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 363 SWITZERLAND GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 364 SWITZERLAND GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 365 SWITZERLAND INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 366 SWITZERLAND INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 367 SWITZERLAND INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 368 SWITZERLAND HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 369 SWITZERLAND HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 370 SWITZERLAND HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 371 SWITZERLAND SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 372 SWITZERLAND SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 373 SWITZERLAND SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 374 SWITZERLAND HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 375 SWITZERLAND HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 376 SWITZERLAND HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 377 SWITZERLAND INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 378 SWITZERLAND TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 379 SWITZERLAND TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 380 SWITZERLAND TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 381 SWITZERLAND MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 382 SWITZERLAND MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 383 SWITZERLAND MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 384 SWITZERLAND CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 385 SWITZERLAND POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 386 SWITZERLAND POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 387 SWITZERLAND CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 388 SWITZERLAND CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 389 SWITZERLAND CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 390 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 391 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 392 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 393 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 394 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 395 SWITZERLAND PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 396 SWITZERLAND PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 397 SWITZERLAND PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 398 SWITZERLAND BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 399 SWITZERLAND BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 400 SWITZERLAND BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 401 SWITZERLAND HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 402 SWITZERLAND HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 403 SWITZERLAND HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 404 SWITZERLAND ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 405 SWITZERLAND ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 406 SWITZERLAND ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 407 SWITZERLAND PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 408 SWITZERLAND PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 409 SWITZERLAND PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 410 SWITZERLAND PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 411 SWITZERLAND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 412 SWITZERLAND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 413 SWITZERLAND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 414 SWITZERLAND COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 415 SWITZERLAND ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 416 SWITZERLAND ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 417 SWITZERLAND ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 418 SWITZERLAND VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 419 SWITZERLAND DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 420 SWITZERLAND DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 421 SWITZERLAND DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 422 SWITZERLAND DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 423 SWITZERLAND RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 424 SWITZERLAND RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 425 SWITZERLAND RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 426 SWITZERLAND URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 427 SWITZERLAND URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 428 SWITZERLAND URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 429 SWITZERLAND CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 430 SWITZERLAND CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 431 SWITZERLAND CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 432 SWITZERLAND INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 433 SWITZERLAND INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 434 SWITZERLAND INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 435 SWITZERLAND FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 436 SWITZERLAND FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 437 SWITZERLAND FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 438 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 439 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 440 SWITZERLAND INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 441 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 442 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 443 SWITZERLAND HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 444 SWITZERLAND PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 445 SWITZERLAND PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 446 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 447 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 448 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 449 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 450 BELGIUM GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 451 BELGIUM GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 452 BELGIUM GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 453 BELGIUM INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 454 BELGIUM INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 455 BELGIUM INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 456 BELGIUM HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 457 BELGIUM HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 458 BELGIUM HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 459 BELGIUM SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 460 BELGIUM SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 461 BELGIUM SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 462 BELGIUM HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 463 BELGIUM HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 464 BELGIUM HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 465 BELGIUM INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 466 BELGIUM TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 467 BELGIUM TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 468 BELGIUM TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 469 BELGIUM MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 470 BELGIUM MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 471 BELGIUM MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 472 BELGIUM CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 473 BELGIUM POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 474 BELGIUM POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 475 BELGIUM CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 476 BELGIUM CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 477 BELGIUM CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 478 BELGIUM BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 479 BELGIUM BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 480 BELGIUM BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 481 BELGIUM BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 482 BELGIUM BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 483 BELGIUM PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 484 BELGIUM PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 485 BELGIUM PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 486 BELGIUM BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 487 BELGIUM BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 488 BELGIUM BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 489 BELGIUM HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 490 BELGIUM HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 491 BELGIUM HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 492 BELGIUM ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 493 BELGIUM ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 494 BELGIUM ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 495 BELGIUM PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 496 BELGIUM PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 497 BELGIUM PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 498 BELGIUM PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 499 BELGIUM FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 500 BELGIUM FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 501 BELGIUM FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 502 BELGIUM COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 503 BELGIUM ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 504 BELGIUM ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 505 BELGIUM ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 506 BELGIUM VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 507 BELGIUM DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 508 BELGIUM DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 509 BELGIUM DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 510 BELGIUM DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 511 BELGIUM RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 512 BELGIUM RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 513 BELGIUM RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 514 BELGIUM URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 515 BELGIUM URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 516 BELGIUM URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 517 BELGIUM CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 518 BELGIUM CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 519 BELGIUM CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 520 BELGIUM INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 521 BELGIUM INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 522 BELGIUM INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 523 BELGIUM FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 524 BELGIUM FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 525 BELGIUM FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 526 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 527 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 528 BELGIUM INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 529 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 530 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 531 BELGIUM HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 532 BELGIUM PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 533 BELGIUM PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 534 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 535 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 536 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 537 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 538 AUSTRIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 539 AUSTRIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 540 AUSTRIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 541 AUSTRIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 542 AUSTRIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 543 AUSTRIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 544 AUSTRIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 545 AUSTRIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 546 AUSTRIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 547 AUSTRIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 548 AUSTRIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 549 AUSTRIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 550 AUSTRIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 551 AUSTRIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 552 AUSTRIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 553 AUSTRIA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 554 AUSTRIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 555 AUSTRIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 556 AUSTRIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 557 AUSTRIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 558 AUSTRIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 559 AUSTRIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 560 AUSTRIA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 561 AUSTRIA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 562 AUSTRIA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 563 AUSTRIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 564 AUSTRIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 565 AUSTRIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 566 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 567 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 568 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 569 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 570 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 571 AUSTRIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 572 AUSTRIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 573 AUSTRIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 574 AUSTRIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 575 AUSTRIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 576 AUSTRIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 577 AUSTRIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 578 AUSTRIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 579 AUSTRIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 580 AUSTRIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 581 AUSTRIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 582 AUSTRIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 583 AUSTRIA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 584 AUSTRIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 585 AUSTRIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 586 AUSTRIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 587 AUSTRIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 588 AUSTRIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 589 AUSTRIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 590 AUSTRIA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 591 AUSTRIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 592 AUSTRIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 593 AUSTRIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 594 AUSTRIA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 595 AUSTRIA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 596 AUSTRIA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 597 AUSTRIA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 598 AUSTRIA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 599 AUSTRIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 600 AUSTRIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 601 AUSTRIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 602 AUSTRIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 603 AUSTRIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 604 AUSTRIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 605 AUSTRIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 606 AUSTRIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 607 AUSTRIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 608 AUSTRIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 609 AUSTRIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 610 AUSTRIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 611 AUSTRIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 612 AUSTRIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 613 AUSTRIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 614 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 615 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 616 AUSTRIA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 617 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 618 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 619 AUSTRIA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 620 AUSTRIA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 621 AUSTRIA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 622 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 623 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 624 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 625 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 626 LUXEMBOURG GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 627 LUXEMBOURG GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 628 LUXEMBOURG GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 629 LUXEMBOURG INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 630 LUXEMBOURG INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 631 LUXEMBOURG INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 632 LUXEMBOURG HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 633 LUXEMBOURG HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 634 LUXEMBOURG HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 635 LUXEMBOURG SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 636 LUXEMBOURG SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 637 LUXEMBOURG SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 638 LUXEMBOURG HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 639 LUXEMBOURG HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 640 LUXEMBOURG HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 641 LUXEMBOURG INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 642 LUXEMBOURG TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 643 LUXEMBOURG TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 644 LUXEMBOURG TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 645 LUXEMBOURG MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 646 LUXEMBOURG MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 647 LUXEMBOURG MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 648 LUXEMBOURG CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 649 LUXEMBOURG POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 650 LUXEMBOURG POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 651 LUXEMBOURG CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 652 LUXEMBOURG CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 653 LUXEMBOURG CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 654 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 655 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 656 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 657 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 658 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 659 LUXEMBOURG PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 660 LUXEMBOURG PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 661 LUXEMBOURG PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 662 LUXEMBOURG BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 663 LUXEMBOURG BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 664 LUXEMBOURG BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 665 LUXEMBOURG HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 666 LUXEMBOURG HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 667 LUXEMBOURG HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 668 LUXEMBOURG ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 669 LUXEMBOURG ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 670 LUXEMBOURG ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 671 LUXEMBOURG PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 672 LUXEMBOURG PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 673 LUXEMBOURG PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 674 LUXEMBOURG PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 675 LUXEMBOURG FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 676 LUXEMBOURG FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 677 LUXEMBOURG FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 678 LUXEMBOURG COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 679 LUXEMBOURG ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 680 LUXEMBOURG ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 681 LUXEMBOURG ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 682 LUXEMBOURG VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 683 LUXEMBOURG DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 684 LUXEMBOURG DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 685 LUXEMBOURG DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 686 LUXEMBOURG DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 687 LUXEMBOURG RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 688 LUXEMBOURG RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 689 LUXEMBOURG RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 690 LUXEMBOURG URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 691 LUXEMBOURG URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 692 LUXEMBOURG URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 693 LUXEMBOURG CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 694 LUXEMBOURG CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 695 LUXEMBOURG CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 696 LUXEMBOURG INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 697 LUXEMBOURG INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 698 LUXEMBOURG INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 699 LUXEMBOURG FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 700 LUXEMBOURG FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 701 LUXEMBOURG FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 702 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 703 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 704 LUXEMBOURG INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 705 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 706 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 707 LUXEMBOURG HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 708 LUXEMBOURG PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 709 LUXEMBOURG PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 710 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
Список рисунков
FIGURE 1 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 2 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: DATA TRIANGULATION
FIGURE 3 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: DROC ANALYSIS
FIGURE 4 GSA POINT-OF-CARE-TESTING (POCT) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 6 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 7 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 11 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE (2024)
FIGURE 12 BEMELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE (2024)
FIGURE 13 GSA POINT-OF-CARE-TESTING (POCT) MARKET: EXECUTIVE SUMMARY
FIGURE 14 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: EXECUTIVE SUMMARY
FIGURE 15 STRATEGIC DECISIONS
FIGURE 16 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES IS EXPECTED TO DRIVE THE GSA POINT-OF-CARE-TESTING (POCT) MARKET, IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 17 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES IS EXPECTED TO DRIVE THE BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 18 GLUCOSE MONITORING PRODUCTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GSA POINT-OF-CARE-TESTING (POCT) MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 19 GLUCOSE MONITORING PRODUCTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE BENELUX POINT-OF-CARE-TESTING (POCT) MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 20 DROC ANALYSIS
FIGURE 21 NUMBER OF PEOPLE USING VARIOUS SUBSTANCES OR EXPERIENCING PROBLEMATIC USE PREVALENCE (PERCENTAGE)
FIGURE 22 NUMBER OF PEOPLE USING VARIOUS SUBSTANCES OR EXPERIENCING PROBLEMATIC USE (IN MILLION)
FIGURE 23 SUBSTANCE USE BEHAVIOURS AMONG 15-YEAR-OLDS IN 2022 (SELF-REPORTED PREVALENCE)
FIGURE 24 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2024
FIGURE 25 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 26 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, CAGR (2025-2032)
FIGURE 27 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 28 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2024
FIGURE 29 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2025 TO 2032 (USD THOUSAND)
FIGURE 30 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, CAGR (2025- 2032)
FIGURE 31 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, LIFELINE CURVE
FIGURE 32 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2024
FIGURE 33 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2025 TO 2032 (USD THOUSAND)
FIGURE 34 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, CAGR (2025- 2032)
FIGURE 35 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, LIFELINE CURVE
FIGURE 36 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2024
FIGURE 37 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 38 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, CAGR (2025- 2032)..
FIGURE 39 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 40 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2024
FIGURE 41 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 42 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, CAGR (2025- 2032)
FIGURE 43 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 45 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2025 TO 2032 (USD THOUSAND)
FIGURE 46 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025- 2032)
FIGURE 47 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2024
FIGURE 49 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 50 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, CAGR (2025-2032)
FIGURE 51 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 52 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2024
FIGURE 53 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2025 TO 2032 (USD THOUSAND)
FIGURE 54 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, CAGR (2025- 2032)
FIGURE 55 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, LIFELINE CURVE
FIGURE 56 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2024
FIGURE 57 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2025 TO 2032 (USD THOUSAND)
FIGURE 58 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, CAGR (2025- 2032)
FIGURE 59 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, LIFELINE CURVE
FIGURE 60 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2024
FIGURE 61 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 62 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, CAGR (2025- 2032)
FIGURE 63 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 64 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2024
FIGURE 65 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 66 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, CAGR (2025- 2032)
FIGURE 67 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 68 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 69 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2025 TO 2032 (USD THOUSAND)
FIGURE 70 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025- 2032)
FIGURE 71 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 72 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: SNAPSHOT (2024)
FIGURE 73 GSA POINT-OF-CARE-TESTING (POCT) MARKET: SNAPSHOT (2024)
FIGURE 74 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY SHARE 2024 (%)

Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.