Asia Pacific Active Medical Implantable Devices Market
市场规模(十亿美元)
CAGR :
% 
 USD
5.06 Billion
USD
11.83 Billion
2025
2033
USD
5.06 Billion
USD
11.83 Billion
2025
2033
| 2026 –2033 | |
| USD 5.06 Billion | |
| USD 11.83 Billion | |
|
|
|
|
亚太有源医疗植入设备市场,按产品(心脏再同步治疗设备 (CRT-D)、植入式心律转复除颤器、植入式心脏起搏器、眼部植入物、神经刺激器、有源植入式听力设备、心室辅助设备、植入式心脏监护仪/可插入式循环记录器、近距离放射治疗、植入式血糖监测仪、下垂足部植入物、肩部植入物、植入式输液泵和植入式配件)、手术类型(传统手术方法和微创手术)、程序(神经血管、心血管、听力等)、最终用户(医院、专科诊所、门诊手术中心、诊所)、国家(日本、中国、澳大利亚、印度、韩国、新加坡、印度尼西亚、泰国、马来西亚、菲律宾、亚太其他地区)行业趋势和预测到 2028 年
市场分析与洞察:亚太地区有源医疗植入设备市场
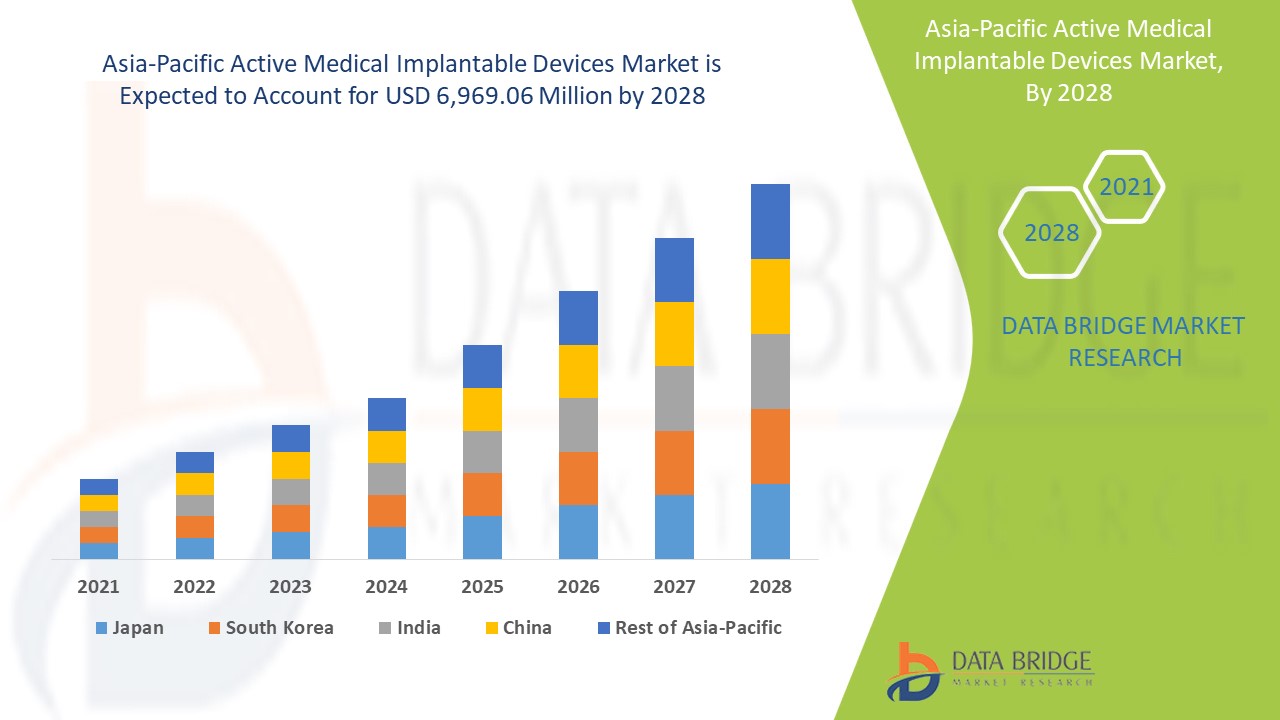
预计亚太地区有源医疗植入设备市场将在 2021 年至 2028 年的预测期内实现显着增长。Data Bridge Market Research 分析称,在 2021 年至 2028 年的预测期内,该市场将以 11.2% 的复合年增长率增长,预计到 2028 年将达到 69.6906 亿美元。心血管等慢性病的增加是预测期内推动市场增长的主要驱动力。然而,植入设备治疗的高成本阻碍了市场的增长。医疗支出的增加为市场增长提供了机会。另一方面,专业工人数量的减少对植入设备市场构成了挑战。
有源医疗植入装置是依靠外部电源而不是身体或重力的装置,这些装置旨在引入体内并留在那里以进行后续程序或治疗。
对于有源医疗植入设备市场,研发的增长以及治疗和产品的进步将影响制造商向市场推出新产品,从而促进其市场增长。目前正在进行各种研究,预计将为制造商创造竞争优势,以开发新的创新型有源医疗植入设备,这有望为有源医疗植入设备市场提供各种其他机会。然而,预计昂贵的治疗和其他住院费用将在预测期内抑制市场增长。
亚太有源医疗植入设备市场报告提供了市场份额、新发展和产品线分析、国内和本地市场参与者的影响的详细信息,分析了新兴收入领域、市场法规变化、产品审批、战略决策、产品发布、地域扩张和市场技术创新方面的机会。要了解分析和市场情况,请联系我们获取分析师简报,我们的团队将帮助您创建收入影响解决方案,以实现您的预期目标。
亚太有源医疗植入设备市场范围和市场规模
根据产品、手术类型、程序和最终用户,亚太有源医疗植入设备市场分为四个显著的部分。
- 根据产品,亚太地区有源医疗植入设备市场细分为心脏再同步治疗设备 (CRT-D)、植入式心律转复除颤器、植入式心脏起搏器、眼部植入物、神经刺激器、有源植入式听力设备、心室辅助设备、植入式心脏监护仪/植入式循环记录器、近距离放射治疗、植入式血糖监测仪、足下植入物、肩部植入物、植入式输液泵和植入式配件。2021 年,由于亚太地区心血管疾病患病率上升,心脏再同步治疗设备 (CRT-D) 细分市场预计将占据市场主导地位。
- 根据手术类型,亚太地区有源医疗植入设备市场分为传统手术方法和微创手术。 2021 年,传统手术方法预计将占据市场主导地位,因为它具有合理的治疗成本和费用。
- 根据程序,亚太有源医疗植入设备市场细分为神经血管、心血管、听力和其他。2021 年,随着心血管疾病的增加,心血管预计将占据市场主导地位。
- 根据最终用户,亚太地区有源医疗植入设备市场分为医院、专科诊所、门诊手术中心和诊所。由于对有源医疗植入设备的需求不断增长,预计 2021 年医院市场将占据主导地位。
有源医疗植入设备市场国家级分析
对有源医疗植入设备市场进行分析,并根据产品、手术类型、程序和最终用户提供市场规模信息。
有源医疗植入设备市场报告涵盖的国家包括日本、中国、澳大利亚、印度、韩国、新加坡、印度尼西亚、泰国、马来西亚、菲律宾和亚太其他地区。
由于有源医疗植入设备制造商数量不断增加,预计亚太地区有源医疗植入设备市场将在 2021 年至 2028 年的预测期内以最高增长率增长。中国引领亚太地区有源医疗植入设备市场的增长,由于该国火灾发生率不断上升,医院市场在该国占据主导地位。
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化会影响市场的当前和未来趋势。新销售、替代销售、国家人口统计、监管法案和进出口关税等数据点是用于预测单个国家市场情景的一些主要指标。此外,在提供国家数据的预测分析时,还考虑了亚太品牌的存在和可用性以及由于来自本地和国内品牌的激烈或稀少的竞争而面临的挑战、销售渠道的影响。
FDA 批准的增加正在推动有源医疗植入设备市场的增长
有源医疗植入设备市场还为您提供每个国家有源医疗植入设备行业增长的详细市场分析,包括有源医疗植入设备药品销售、有源医疗植入设备技术进步的影响以及监管环境的变化及其对市场的支持。数据涵盖 2010 年至 2018 年的历史时期。
竞争格局和有源医疗植入设备市场份额分析
有源医疗植入设备市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、收入、市场潜力、研发投资、新市场计划、生产基地和设施、公司优势和劣势、产品发布、产品试验渠道、产品批准、专利、产品宽度和广度、应用优势、技术生命线曲线。以上提供的数据点仅与公司对有源医疗植入设备市场的关注有关。
在亚太有源医疗植入设备市场开展业务的主要公司包括 NeuroPace, Inc.、Axonics, Inc.、Stimwave LLC、NEVRO CORP、Second Sight、BIOTRONIK、ABIOMED、Boston Scientific Corporation、Medtronic、Abbott、Eckert & Ziegler.、Sonova、浙江诺尔康生物技术有限公司、Demant A/S、Cochlear Ltd、Microson、Oticon Medical、Nano Retina、GluSense、MED-EL Medical Electronics 等。
全球各地的公司还发起了多项产品发布和协议,这也加速了有源医疗植入设备市场的增长。
例如,
- 2021年1月,波士顿科学宣布已达成收购Preventice Solutions, Inc.的协议,此次收购将帮助该公司扩大其心脏产品组合。
- 2021 年 7 月,雅培宣布在美国推出可插入式心脏监护仪 Jot Dx。该设备可帮助临床医生查看异常心律,并可进行远程检测,提高心律失常的诊断准确率。这将有助于该公司在未来几年进一步占领市场
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。