Asia Pacific Vaccines Market
市场规模(十亿美元)
CAGR :
% 
 USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
| 2023 –2030 | |
| USD 9,048.51 Million | |
| USD 18,031.69 Million | |
|
|
|
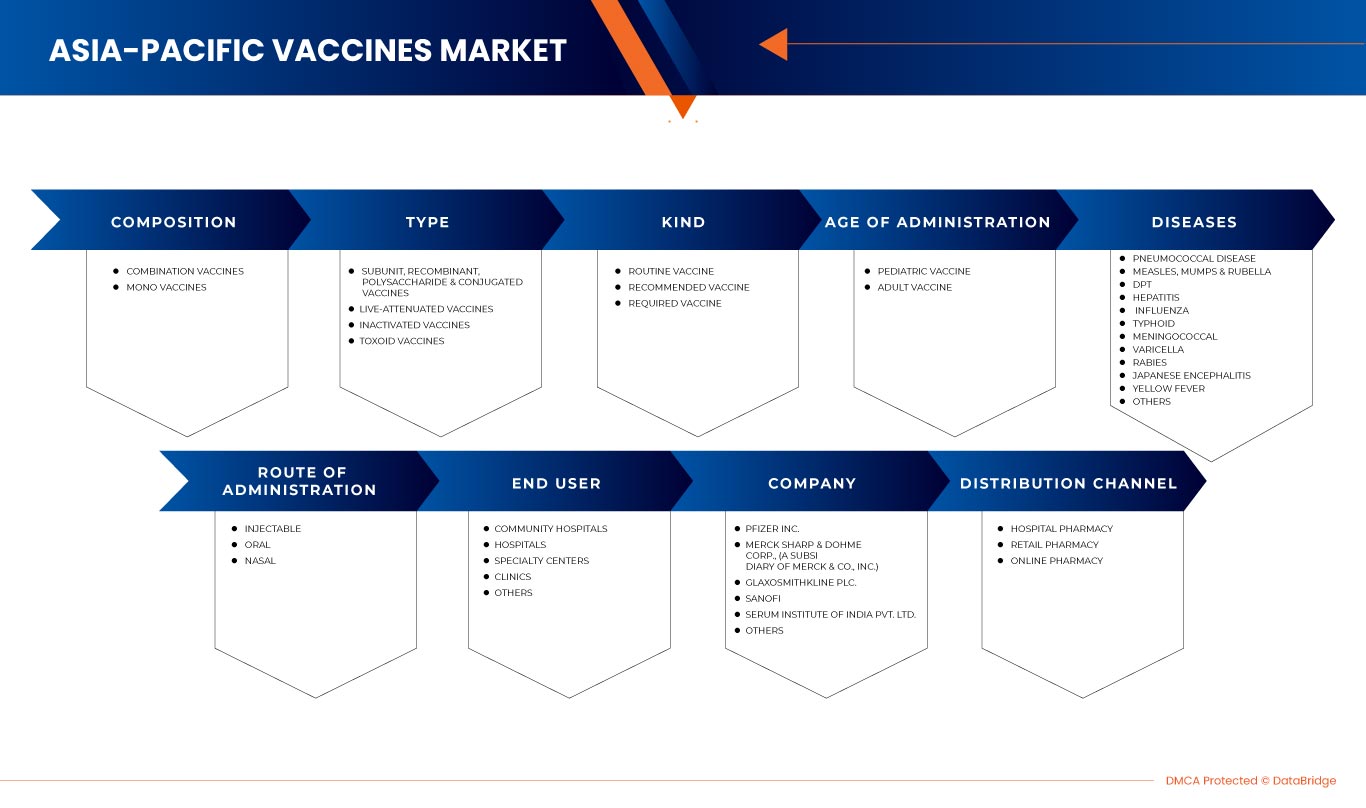
Asia-Pacific Vaccines Market, By Composition (Combination Vaccines and Mono Vaccines), Type (Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines, Live-Attenuated Vaccines, Inactivated Vaccines, and Toxoid Vaccines), Kind (Routine Vaccine, Recommended Vaccine, and Required Vaccine), Age of Administration (Pediatric Vaccine and Adult Vaccine), Diseases (Pneumococcal Disease, Measles, Mumps & Varicella, DPT, Hepatitis, Influenza, Typhoid, Meningococcal, Rabies, Japanese Encephalitis, Yellow Fever, and Others), Route of Administration (Injectable, Oral, and Nasal), End User (Community Hospitals, Hospitals, Specialty Centres, Clinics, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy) - Industry Trends and Forecast to 2030.
Asia-Pacific Vaccines Market Analysis and Insights
The increasing prevalence of infectious diseases, including bacterial and viral diseases, provides the market with lucrative growth. Along with this, increasing government support and launching newer vaccines are also boosting the vaccine market. Another factor boosting the vaccine market growth is increasing vaccination awareness and demand for effective COVID-19 vaccines.
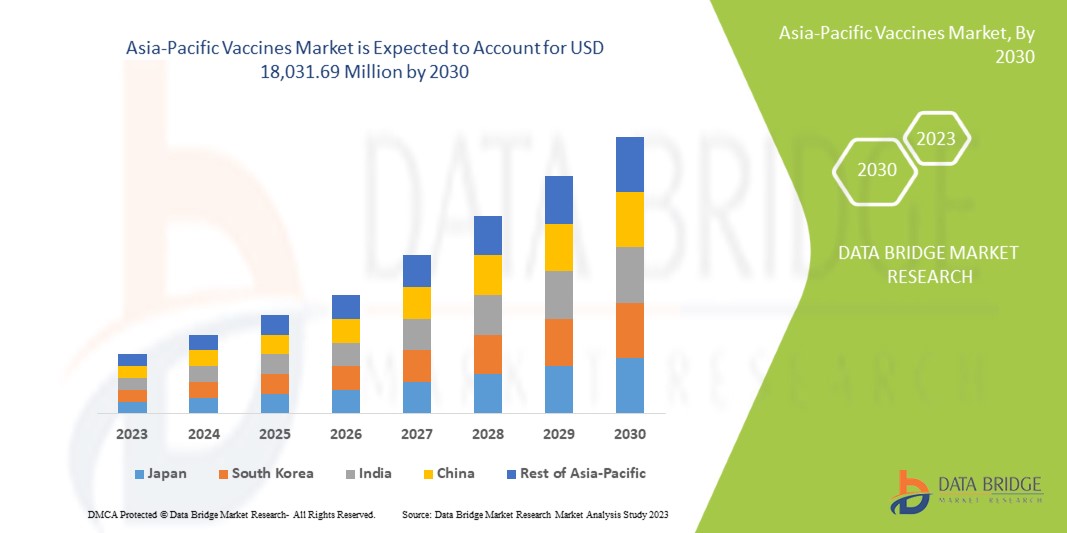
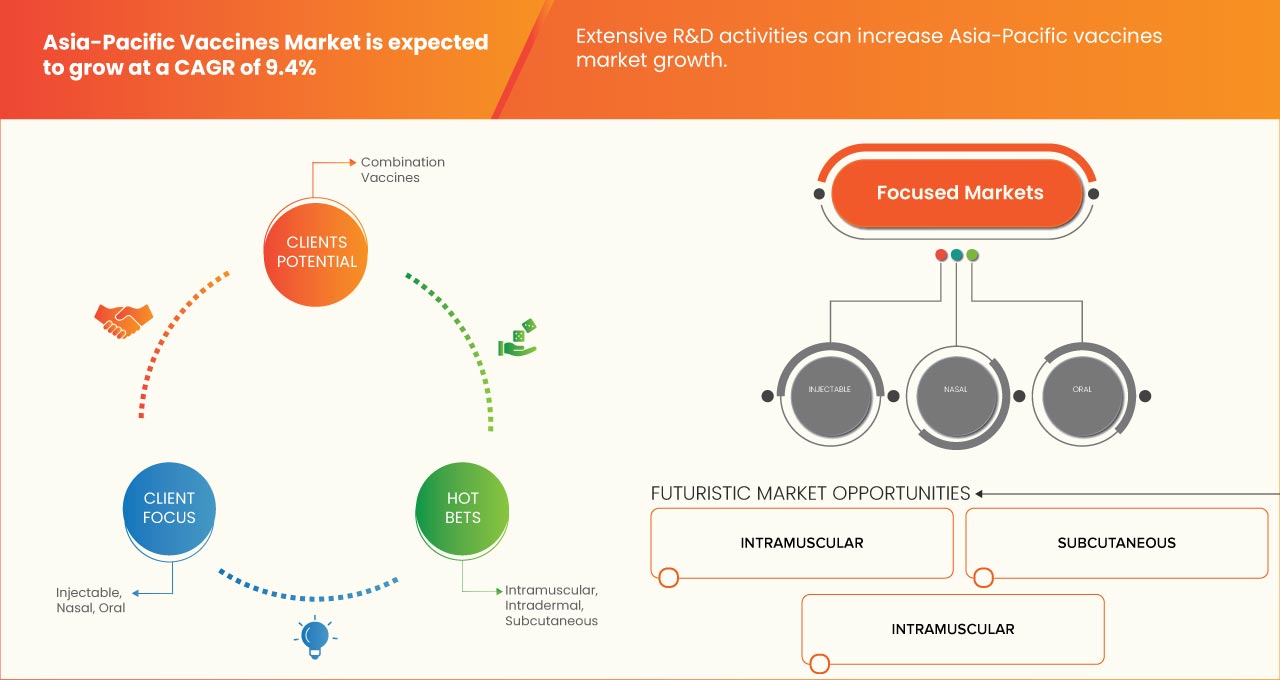
The Asia-Pacific vaccines market is expected to grow in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 9.4% in the forecast period of 2023 to 2030 and is expected to reach USD 18,031.69 Million by 2030 from USD 9,048.51 million in 2022.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Composition (Combination Vaccines and Mono Vaccines), Type (Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines, Live-Attenuated Vaccines, Inactivated Vaccines, and Toxoid Vaccines), Kind (Routine Vaccine, Recommended Vaccine, and Required Vaccine), Age of Administration (Pediatric Vaccine and Adult Vaccine), Diseases (Pneumococcal Disease, Measles, Mumps & Varicella, DPT, Hepatitis, Influenza, Typhoid, Meningococcal, Rabies, Japanese Encephalitis, Yellow Fever, and Others), Route of Administration (Injectable, Oral, and Nasal), End User (Community Hospitals, Hospitals, Specialty Centres, Clinics, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy) |
|
Countries Covered |
Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Vietnam, and Rest of Asia-Pacific |
|
Market Players Covered |
Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Pvt. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (a subsidiary of Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (a subsidiary of Johnson & Johnson Services, Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd, and BAXTER VACCINES (a subsidiary of Baxter), among others |
Market Definition of Asia-Pacific Vaccines Market
Vaccines are product that stimulates the individual immune system to induce immunity against a particular disease. Vaccines work on the principle of memory and recognition. When weakened or killed microbes are injected into a body, these microbes cause B cells, memory cells of the immune system, to recognize the pathogen. In the future, if the same pathogen attacks the body, it works against those. Vaccines have been discovered for infectious diseases, including pneumococcal disease, measles, mumps, rubella, hepatitis, influenza, typhoid, varicella, and rabies.
Vaccines are of two types: combination vaccines (containing different strains of the pathogen) and monovaccines (containing a single strain of the pathogen). Different kinds of vaccines have been developed based on the material extracted from the pathogen, which can be polysaccharide coat, DNA, RNA, and the whole organism, either inactivated or live.
These are the vaccines that resulted in the eradication of diseases such as polio. As per the preference and efficiency of vaccines, these can be injected via several routes of administration that can be injectable, oral, or nasal. However, the injectable route of vaccine administration is highly preferred as it induces a systemic response. Vaccination can be achieved at hospitals, community clinics, and specialty clinics, among others, by trained personnel having appropriate knowledge about vaccine administration devices.
The increasing prevalence of infectious diseases, including bacterial and viral diseases, provides the market with lucrative growth. Along with this, increasing government support and the launch of newer vaccines also boost the vaccine market. Another factor boosting the vaccine market growth is increasing vaccination awareness and demand for effective COVID-19 vaccines.
Asia-Pacific Vaccines Market Dynamics
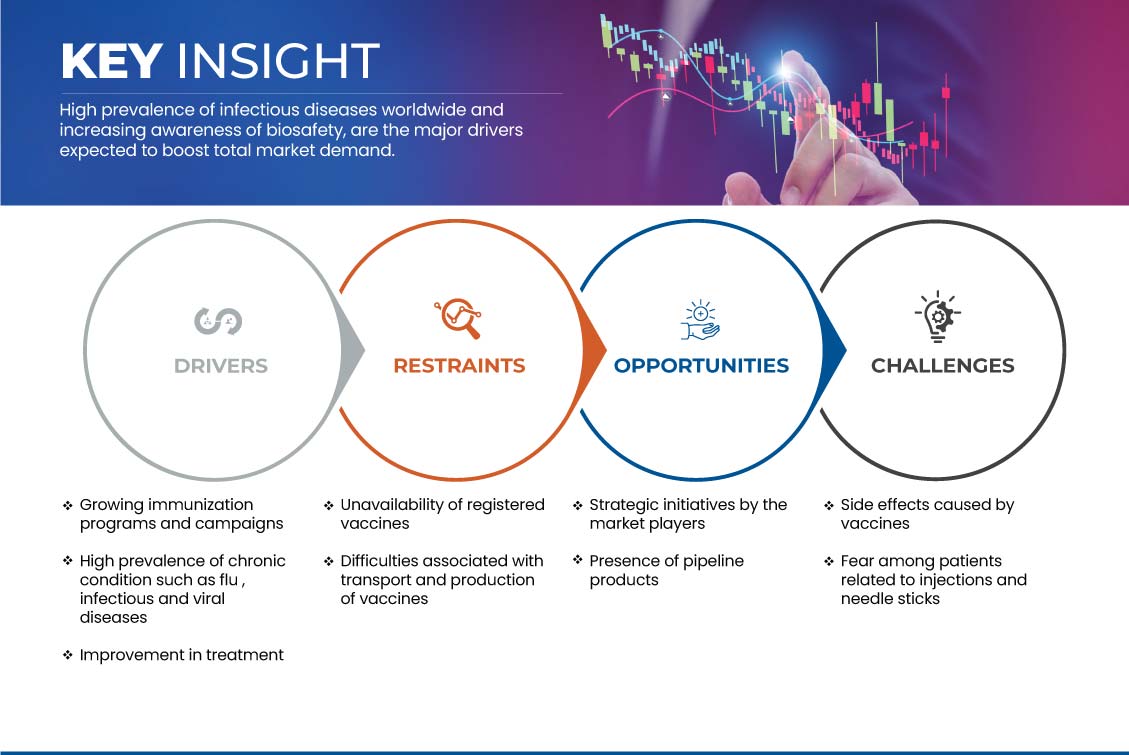
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
DRIVERS
GROWTH IN THE IMMUNIZATION PROGRAMS AND CAMPAIGNS
由于慢性病数量不断增加,全球范围内的免疫接种计划和活动也在不断增加。由于肝炎、白喉、百日咳和脊髓灰质炎等传染病在环境中盛行,迫切需要提高人们对疫苗接种的认识,这可以通过开展多项活动和计划来实现。据报道,随着传染病的增加,免疫接种计划的数量也在增加。全球范围内的免疫覆盖率也在增加,旨在对抗使人衰弱的疾病。
然而,仍有约 2000 万人未接种疫苗或接种不足,疫苗接种需求巨大。全球慢性病的患病率正在上升。因此,接种疫苗的需求很大。这意味着,免疫计划和运动的不断发展有望推动疫苗市场的增长。
流感、传染病和病毒性疾病等慢性病患病率高
传染病的流行率在世界范围内不断上升,流感和细菌性传染病正在迅速增加。传染病的发病率不断上升,需要通过接种疫苗或免疫来预防疾病。由于疾病的增加,迫切需要大规模接种疫苗。大规模接种疫苗需要针对多种疾病,因此预计将为疫苗市场带来丰厚的增长。
随着疾病病例的增加,为了防止人口规模扩大,大规模疫苗接种的关注度也在增加。全球许多年轻时未接种疫苗的人都接种了疫苗。因此,为了满足这些需求,对新型疫苗的需求正在增加,因此有望成为亚太疫苗市场的推动力。
克制
无法获得注册疫苗
疫苗的监管审批严格,开发程序耗时,是导致无法获得注册疫苗的部分原因。监管机构在疫苗评估、许可、控制和监督方面遇到困难。这些规定导致全球疫苗供应延迟。
因此,不同国家制造公司的监管流程和文件形成可能会起到制约作用,阻碍疫苗市场的增长。
机会
市场参与者的战略举措
市场参与者在疫苗市场采取各种战略举措,包括扩张、合作和收购。这些举措使他们能够增加公司的产品组合,从而扩大市场并提高客户对产品的需求,最终使市场参与者获得最大收益。
随着全球对有效和新型疫苗的需求不断增加,顶级市场参与者采取的这些战略举措旨在加强业务运营并在市场上获得更多盈利。
市场参与者采取的各种战略举措使他们能够扩大疫苗业务并获得更多市场增长。因此,从事疫苗业务的市场参与者正在采取多项战略举措,预计这些举措将成为疫苗市场增长的机会。
挑战
疫苗引起的副作用
疫苗是一种有助于预防不同疾病的医疗产品。但有时,使用疫苗会产生副作用。其中一些是轻微的副作用,如注射部位发红、疼痛或肿胀。但疫苗的不良副作用很少见,但会危及生命。
疫苗副作用可能会危及生命,引起民众恐慌,并影响疫苗生产商的信誉,影响产品销量,因此疫苗副作用可能会阻碍疫苗市场的增长。
最新动态
- 2022 年 10 月,印度尼西亚推出了首款国产 COVID-19 疫苗。IndoVac 疫苗由印度尼西亚国有制药公司 Bio Farma 和位于德克萨斯州休斯顿的独立健康科学中心贝勒医学院联合开发
- 2020 年 11 月,默克公司子公司 Merck Sharp & Dohme Corp. 签署协议,收购临床阶段生物制药公司 OncoImmune。OncoImmune 公司高度专注于 COVID-19 治疗方案的开发。通过这项协议,该公司有望开发新的候选疫苗
- 2020 年 9 月,赛诺菲和葛兰素史克与加拿大政府签署了一项协议,供应 7200 万剂 COVID-19 疫苗。COVID-19 疫苗的需求量很大,而且随着疫情的加剧,需求也在增加。这项协议使该公司能够确保未来的潜在
亚太疫苗市场范围
亚太疫苗市场细分为成分、类型、种类、给药年龄、疾病、给药途径、最终用户和分销渠道。这些细分市场之间的增长将帮助您分析行业中增长微弱的细分市场,并为用户提供有价值的市场概览和市场洞察,以做出战略决策,确定核心市场应用。
作品
- 联合疫苗
- 单药疫苗
根据构成,亚太疫苗市场分为联合疫苗和单一疫苗。
类型
- 亚单位、重组、多糖和结合疫苗
- 减毒活疫苗
- 灭活疫苗
- 类毒素疫苗
根据类型,亚太疫苗市场分为亚单位疫苗、重组疫苗、多糖疫苗、结合疫苗、减毒活疫苗、灭活疫苗和类毒素疫苗。
种类
- 常规疫苗
- 推荐疫苗
- 必需疫苗
根据种类,亚太疫苗市场分为常规疫苗、推荐疫苗和必需疫苗。
执政年龄
- 儿童疫苗
- 成人疫苗
根据接种年龄,亚太疫苗市场分为儿童疫苗和成人疫苗
疾病
- 肺炎球菌病
- 麻疹、腮腺炎和水痘
- 德普特
- 肝炎
- 流感
- 伤寒
- 脑膜炎球菌
- 水痘
- 狂犬病
- 日本脑炎
- 黄热病
- 其他的
根据疾病,亚太疫苗市场分为肺炎球菌病、麻疹、腮腺炎和水痘、百日咳、肝炎、流感、伤寒、脑膜炎、狂犬病、日本脑炎、黄热病等。
给药途径
- 注射剂
- 鼻腔
- 口服
根据给药途径,亚太疫苗市场分为注射疫苗、口服疫苗和鼻腔疫苗。
最终用户
- 社区医院
- 医院
- 专科中心
- 诊所
- 其他的
根据最终用户,亚太疫苗市场分为社区医院、医院、专科中心、诊所和其他。
分销渠道
- 医院药房
- 零售药店
- 网上药店
根据分销渠道,亚太疫苗市场分为医院药房、零售药房和网上药房。
亚太疫苗市场区域分析/见解
对亚太疫苗市场进行了分析,并按国家、构成、类型、种类、接种年龄、疾病、接种途径、最终用户和分销渠道提供了市场规模见解和趋势,如上所述。
本市场报告涵盖的国家包括日本、中国、韩国、印度、澳大利亚、新加坡、泰国、马来西亚、印度尼西亚、菲律宾、越南和亚太地区其他国家。
预计日本将在市场份额和收入方面主导亚太疫苗市场,并将在预测期内继续保持主导地位。这是由于人们对预防性健康检查的偏好日益增加。
报告的国家部分还提供了影响市场当前和未来趋势的各个市场影响因素和市场法规变化。新旧销售、国家人口统计、疾病流行病学和进出口关税等数据点是预测各个国家市场情景的一些主要指标。此外,在对国家数据进行预测分析时,还考虑了全球品牌的存在和可用性以及它们因来自本地和国内品牌的竞争而面临的挑战以及销售渠道的影响。
竞争格局和亚太疫苗市场份额分析
疫苗市场竞争格局提供了竞争对手的详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、全球影响力、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度以及应用主导地位。以上数据点仅与公司对疫苗市场的关注有关。
亚太疫苗市场的一些主要参与者包括 Bharat Biotech、Biological E Limited、Bio Farma、Serum Institute of India Pvt. Ltd.、武田制药有限公司、默克夏普和多姆公司(默克公司的子公司)、雅培、阿斯利康、赛诺菲、辉瑞公司、杨森全球服务有限责任公司(强生服务公司的子公司)、F. Hoffmann-La Roche Ltd、Panacea Biotec Ltd 和 BAXTER VACCINES(百特的子公司)等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC VACCINES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 COMPOSITION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL MODEL
4.2 PORTER'S FIVE FORCES
4.3 EPIDEMIOLOGY
4.4 INDUSTRIAL INSIGHTS:
4.5 PIPELINE ANALYSIS
4.6 ASIA-PACIFIC VACCINES MARKET: SUPPLY CHAIN MANAGEMENT OF VACCINES
4.6.1 COLD CHAIN STORAGE:
4.6.2 PROCESS OF LOGISTICS
5 REGULATORY FRAMEWORK
5.1 JAPAN
5.2 CHINA
5.3 SOUTH KOREA
5.4 INDIA
5.5 AUSTRALIA
5.6 SINGAPORE
5.7 THAILAND
5.8 MALAYSIA
5.9 INDONESIA
5.1 VIETNAM
5.11 PHILIPPINES
5.12 REST OF ASIA-PACIFIC
5.12.1 TAIWAN
5.12.2 CAMBODIA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS
6.1.2 HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU, INFECTIOUS AND VIRAL DISEASES
6.1.3 IMPROVEMENT IN TREATMENT
6.1.4 LAUNCH OF NEWER VACCINES
6.1.5 INCREASING GOVERNMENT SUPPORT
6.2 RESTRAINTS
6.2.1 UNAVAILABILITY OF REGISTERED VACCINES
6.2.2 DIFFICULTIES ASSOCIATED WITH THE TRANSPORT AND PRODUCTION OF VACCINES
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.3.2 PRESENCE OF PIPELINE PRODUCTS
6.3.3 RISE IN EXPENDITURE IN THE HEALTHCARE SECTOR
6.3.4 INCREASING AWARENESS FOR VACCINATION
6.4 CHALLENGES
6.4.1 SIDE EFFECTS CAUSED BY VACCINES
6.4.2 FEAR AMONG PATIENTS RELATED TO INJECTIONS AND NEEDLE STICKS
6.4.3 PRODUCT RECALL
7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION
7.1 OVERVIEW
7.2 COMBINATION VACCINES
7.3 MONO VACCINES
8 ASIA-PACIFIC VACCINES MARKET, BY TYPE
8.1 OVERVIEW
8.2 SUBUNIT, RECOMBINANT, POLYSACCHARIDE, AND CONJUGATE VACCINES
8.2.1 PNEUMOCOCCAL DISEASE
8.2.2 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
8.2.3 HPV (HUMAN PAPILLOMA VIRUS)
8.2.4 HEPATITIS B
8.2.5 MENINGOCOCCAL
8.2.6 SHINGLES
8.2.7 WHOOPING COUGH
8.2.8 OTHERS
8.3 LIVE-ATTENUTAED VACCINES
8.3.1 ROTAVIRUS
8.3.2 MEASLES
8.3.3 MUMPS
8.3.4 RUBELLA
8.3.5 SMALLPOX
8.3.6 YELLOW FEVER
8.3.7 OTHERS
8.4 INACTIVATED VACCINES
8.4.1 FLU (SHOT ONLY)
8.4.2 POLIO (SHOT ONLY)
8.4.3 HEPATITIS A
8.4.4 RABIES
8.4.5 OTHERS
8.5 TOXOID VACCINES
8.5.1 DIPHTHERIA, TETANUS & PERTUSSIS (DTP)
8.5.2 OTHERS
9 ASIA-PACIFIC VACCINES MARKET, BY KIND
9.1 OVERVIEW
9.2 ROUTINE VACCINES
9.2.1 PNEUMOCOCCAL DISEASES
9.3 DIPTHERIA, TETANUS & PERTUSIS(DPT)
9.3.1 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
9.3.2 MEASLES
9.3.3 MUMPS
9.3.4 HEPATITIS B
9.3.5 RUBELLA
9.3.6 POLIO
9.3.7 OTHERS
9.4 RECOMMENDED VACCINE
9.4.1 TYPHOID FEVER VACCINE
9.5 HEPATITIS A
9.5.1 RABIES
9.5.2 JAPANESE ENCEPHALITIS
9.5.3 TICK-BORNE ENCEPHALITIS
9.5.4 CHOLERA
9.5.5 OTHERS
9.6 REQUIRED VACCINE
9.6.1 MENINGOCOCCAL
9.7 YELLOW FEVER
9.7.1 OTHERS
10 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION
10.1 OVERVIEW
10.2 PEDIATRIC VACCINE
10.2.1 PNEUMOCOCCAL DISEASES
10.3 MEASLES, MUMPS & RUBELLA
10.3.1 DIPTHERIA, TETANUS & PERTUSIS (DPT)
10.3.2 ROTAVIRUS
10.3.3 MENINGOCOCCAL
10.3.4 VARICELLA
10.3.5 POLIO
10.3.6 TUBERCULOSIS
10.3.7 MALARIA
10.3.8 OTHERS
10.4 ADULT VACCINE
10.4.1 INFLUENZA
10.5 HPV (HUMAN PAPILLOMA VIRUS)
10.5.1 TYPHOID
10.5.2 HEPATITIS B
10.5.3 JAPANESE ENCEPHALITIS
10.5.4 YELLOW FEVER
10.5.5 CANCER
10.5.6 OTHERS
11 ASIA-PACIFIC VACCINES MARKET, BY DISEASES
11.1 OVERVIEW
11.2 PNEUMOCCOCAL DISEASE
11.3 MEASLES, MUMPS & RUBELLA
11.4 DPT
11.5 HEPATITIS
11.6 INFLUENZA
11.7 TYPHOID
11.8 MENINGOCOCCAL
11.9 VARICELLA
11.1 RABIES
11.11 JAPANESE ENCEPHALITIS
11.12 YELLOW FEVER
11.13 OTHERS
12 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 INJECTABLE
12.2.1 INTRAMUSCULAR
12.2.2 SUBCUTANEOUS
12.2.3 INTRADERMAL
12.3 ORAL
12.4 NASAL
13 ASIA-PACIFIC VACCINES MARKET, BY END USER
13.1 OVERVIEW
13.2 COMMUNITY HOSPITALS
13.3 HOSPITALS
13.4 SPECIALTY CENTERS
13.5 CLINICS
13.6 OTHERS
14 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 ASIA-PACIFIC VACCINE MARKET
15.1 ASIA-PACIFIC
15.1.1 JAPAN
15.1.2 CHINA
15.1.3 AUSTRALIA
15.1.4 INDIA
15.1.5 SOUTH KOREA
15.1.6 SINGAPORE
15.1.7 MALAYSIA
15.1.8 THAILAND
15.1.9 INDONESIA
15.1.10 PHILIPPINES
15.1.11 VIETNAM
15.1.12 REST OF ASIA PACIFIC
16 ASIA-PACIFIC VACCINES MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
16.2 DISEASE SHARE ANALYSIS: PFIZER, INC.
16.3 COUNTRY SHARE ANALYSIS: PFIZER, INC.
16.4 DISEASE SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.5 COUNTRY SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.6 DISEASE SHARE ANALYSIS: GLAXOSMITHKLINE PLC
16.7 COUNTRY SHARE ANALYSIS: GLAXOSMITHKLINE PLC.
16.8 DISEASE SHARE ANALYSIS: SANOFI
16.9 COUNTRY SHARE ANALYSIS: SANOFI
16.1 DISEASE SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
16.11 COUNTRY SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 PFIZER INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY WEBSITE AND PRESS RELEASES
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GLAXOSMITHKLINE PLC.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 PRODUCT PORTFOLIO
18.3.4 RECENT DEVELOPMENTS
18.4 SANOFI
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 PRODUCT PORTFOLIO
18.4.4 RECENT DEVELOPMENTS
18.5 SERUM INSTITUTE OF INDIA PVT. LTD.
18.5.1 COMPANY SNAPSHOT
18.5.2 PRODUCT PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 ASTRAZENECA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 ALK
18.8.1 COMPANY SNAPSHOT
18.8.2 REVENUE ANALYSIS
18.8.3 PRODUCT PORTFOLIO
18.8.4 RECENT DEVELOPMENTS
18.9 BAXTER VACCINES (A SUBSIDIARY OF BAXTER)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BHARAT BIOTECH
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENTS
18.11 BIO FARMA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 BIOLOGICAL E LIMITED
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 DAIICHI SANKYO COMPANY, LIMITED
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 F. HOFFMANN-LA ROCHE LTD
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 JANSSEN GLOBAL SERVICES, LLC (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC.)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENTS
18.16 LANZHOU BIOLOGICAL PRODUCTS RESEARCH INSTITUTE CO., LTD.,
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENTS
18.17 PANACEA BIOTEC LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENTS
18.18 SEQIRUS (A SUBSIDIARY OF CSL LIMITED)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 TAKEDA PHARMACEUTICAL COMPANY LIMITED
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 COMPANY WEBSITE AND PRESS RELEASES
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
表格列表
TABLE 1 ASIA-PACIFIC VACCINES MARKET, PIPELINE ANALYSIS
TABLE 2 RECOMMENDED TEMPERATURE AND STORAGE LENGTH AT VARIOUS LEVELS OF THE COLD CHAIN.
TABLE 3 LOGISTICS PROCESS ACROSS DIFFERENT REGIONS.
TABLE 4 LAWS AND REGULATIONS IN TAIWAN
TABLE 5 VACCINES UNDER CLINICAL TRIAL
TABLE 6 THE SIDE EFFECTS RELATED TO THE VACCINES
TABLE 7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 8 ASIA-PACIFIC VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA-PACIFIC SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 ASIA-PACIFIC LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA-PACIFIC INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 ASIA-PACIFIC TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 ASIA-PACIFIC VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 14 ASIA-PACIFIC ROUTINE VACCINES IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 15 ASIA-PACIFIC RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 16 ASIA-PACIFIC REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 17 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 18 ASIA-PACIFIC PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 19 ASIA-PACIFIC ADULT VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 20 ASIA-PACIFIC VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 21 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 22 ASIA-PACIFIC INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 23 ASIA-PACIFIC VACCINES MARKET, BY END USER, 2022-2030 (USD MILLION)
TABLE 24 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 25 ASIA-PACIFIC KNEE CARTILAGE REPAIR MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 26 JAPAN VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 27 JAPAN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 JAPAN LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 JAPAN INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 JAPAN TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 33 JAPAN ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 34 JAPAN RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 35 JAPAN REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 36 JAPAN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 37 JAPAN PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 JAPAN ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 JAPAN VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 40 JAPAN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 JAPAN INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 JAPAN VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 43 JAPAN VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 44 CHINA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 45 CHINA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 46 CHINA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 47 CHINA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 CHINA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 49 CHINA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 50 CHINA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 51 CHINA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 52 CHINA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 53 CHINA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 54 CHINA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 55 CHINA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 56 CHINA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 57 CHINA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 58 CHINA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 59 CHINA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 60 CHINA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 61 CHINA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 AUSTRALIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 63 AUSTRALIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 64 AUSTRALIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 65 AUSTRALIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 66 AUSTRALIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 AUSTRALIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 AUSTRALIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 69 AUSTRALIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 70 AUSTRALIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 71 AUSTRALIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 72 AUSTRALIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 73 AUSTRALIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 74 AUSTRALIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 AUSTRALIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 76 AUSTRALIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 77 AUSTRALIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 78 AUSTRALIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 79 INDIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 80 INDIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 INDIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 INDIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 INDIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 INDIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 INDIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 86 INDIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 87 INDIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 88 INDIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 89 INDIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 90 INDIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 91 INDIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 92 INDIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 93 INDIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 94 INDIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 95 INDIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 96 INDIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 97 SOUTH KOREA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 98 SOUTH KOREA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 SOUTH KOREA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 100 SOUTH KOREA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 101 SOUTH KOREA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 SOUTH KOREA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 103 SOUTH KOREA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 104 SOUTH KOREA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 105 SOUTH KOREA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 106 SOUTH KOREA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 107 SOUTH KOREA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 108 SOUTH KOREA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 109 SOUTH KOREA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 110 SOUTH KOREA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SOUTH KOREA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SOUTH KOREA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SOUTH KOREA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 SINGAPORE VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 115 SINGAPORE SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 SINGAPORE LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 SINGAPORE INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 SINGAPORE TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 SINGAPORE VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 120 SINGAPORE ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 121 SINGAPORE RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 122 SINGAPORE REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 123 SINGAPORE VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 124 SINGAPORE PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 125 SINGAPORE ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 126 SINGAPORE VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 127 SINGAPORE VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 128 SINGAPORE INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 129 SINGAPORE VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 SINGAPORE VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 131 MALAYSIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 132 MALAYSIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 MALAYSIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 MALAYSIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 135 MALAYSIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 136 MALAYSIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 137 MALAYSIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 138 MALAYSIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 139 MALAYSIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 140 MALAYSIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 141 MALAYSIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 142 MALAYSIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 143 MALAYSIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 144 MALAYSIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 145 MALAYSIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 146 MALAYSIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 MALAYSIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 148 MALAYSIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 149 THAILAND VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 150 THAILAND VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 THAILAND SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 THAILAND LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 THAILAND INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 THAILAND TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 THAILAND VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 156 THAILAND RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 157 THAILAND REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 158 THAILAND VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 THAILAND PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 THAILAND ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 161 THAILAND VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 162 THAILAND VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 163 THAILAND INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 164 THAILAND VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 THAILAND VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 INDONESIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 167 INDONESIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 INDONESIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 INDONESIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 INDONESIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 171 INDONESIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 172 INDONESIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 173 INDONESIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 174 INDONESIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 175 INDONESIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 176 INDONESIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 177 INDONESIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 178 INDONESIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 179 INDONESIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 180 INDONESIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 181 INDONESIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 182 INDONESIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 183 INDONESIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 184 PHILIPPINES VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 185 PHILIPPINES VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 186 PHILIPPINES SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 187 PHILIPPINES LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 PHILIPPINES INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 PHILIPPINES TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 PHILIPPINES VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 191 PHILIPPINES ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 192 PHILIPPINES RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 193 PHILIPPINES REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 194 PHILIPPINES VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 PHILIPPINES PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 PHILIPPINES ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 197 PHILIPPINES VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 198 PHILIPPINES VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 199 PHILIPPINES INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 200 PHILIPPINES VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 201 PHILIPPINES VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 202 VIETNAM VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 203 VIETNAM VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 VIETNAM SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 VIETNAM LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 VIETNAM INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 207 VIETNAM TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 208 VIETNAM VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 209 VIETNAM ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 210 VIETNAM RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 211 VIETNAM REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 212 VIETNAM VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 213 VIETNAM PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 214 VIETNAM ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 215 VIETNAM VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 216 VIETNAM VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 217 VIETNAM INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 218 VIETNAM VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 219 VIETNAM VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 220 REST OF ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
图片列表
FIGURE 1 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC VACCINES MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC VACCINES MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC VACCINES MARKET: ASIA-PACIFIC VS COUNTRY MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC VACCINES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC VACCINES MARKET: MULTIVARIATE MODELLING
FIGURE 7 ASIA-PACIFIC VACCINES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 ASIA-PACIFIC VACCINES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 10 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS AND THE HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU AND BACTERIAL INFECTIOUS DISEASES ARE DRIVING THE ASIA-PACIFIC VACCINES MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 11 THE COMBINATION VACCINES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC VACCINES MARKET IN 2023 & 2030
FIGURE 12 FDA REGULATORY REVIEW PROCESS OF VACCINES
FIGURE 13 PROCESS OF SPECIAL APPROVAL ON VACCINES DURING THE 2019 H1N1PDM PANDEMIC
FIGURE 14 REGULATION OVERVIEW FOR THERAPEUTICS IN SINGAPORE
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF THE ASIA-PACIFIC VACCINES MARKET
FIGURE 16 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2022
FIGURE 17 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2023-2030 (USD MILLION)
FIGURE 18 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, CAGR (2023-2030)
FIGURE 19 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, LIFELINE CURVE
FIGURE 20 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2022
FIGURE 21 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA-PACIFIC VACCINES MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 23 ASIA-PACIFIC VACCINES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2022
FIGURE 25 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2023-2030 (USD MILLION)
FIGURE 26 ASIA-PACIFIC VACCINES MARKET: BY KIND, CAGR (2023-2030)
FIGURE 27 ASIA-PACIFIC VACCINES MARKET: BY KIND, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2022
FIGURE 29 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 30 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 31 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2022
FIGURE 33 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 34 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 35 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 37 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 38 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 39 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2022
FIGURE 41 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 ASIA-PACIFIC VACCINES MARKET: BY END USER, CAGR (2023-2030)
FIGURE 43 ASIA-PACIFIC VACCINES MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC VACCINE MARKET: SNAPSHOT (2022)
FIGURE 49 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022)
FIGURE 50 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 ASIA-PACIFIC VACCINE MARKET: BY COMPOSITION (2023-2030)
FIGURE 53 ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 54 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 55 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 56 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 57 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 58 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 59 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 60 SANOFI, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 61 SANOFI ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 62 SERUM INSTITUTE OF INDIA PVT. LTD., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 63 SERUM INSTITUTE OF INDIA PVT. LTD. ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。