Europe Lysosomal Storage Disorder Drugs Market
市场规模(十亿美元)
CAGR :
% 
 USD
2.92 Billion
USD
5.68 Billion
2024
2032
USD
2.92 Billion
USD
5.68 Billion
2024
2032
| 2025 –2032 | |
| USD 2.92 Billion | |
| USD 5.68 Billion | |
|
|
|
欧洲溶酶体贮积症药物市场细分,按疾病类型戈谢病法布里病庞贝病、粘多糖贮积症 (MPS)、尼曼匹克病克拉伯病等)、类型(酶替代疗法 (ERT)、底物减少疗法 (SRT)、伴侣疗法等)、药物(伊米苷酶、阿加糖苷酶 Beta、艾杜尔硫酸酶、阿糖苷酶 Alpha、维拉苷酶、塔利苷酶 Alfa、Laronidase、阿加糖苷酶 Alpha、Galsulfase、阿伐葡萄糖苷酶 Alfa 等)、给药途径(静脉 (IV)、皮下 (SC)、口服等)、年龄组(儿童、成人和老年人)、性别(男性和女性)、分销渠道(医院药房、药店和零售)药店和网上药店)- 行业趋势和 2032 年预测
溶酶体储存障碍药物市场分析
欧洲溶酶体贮积症药物市场涵盖了用于诊断、治疗和管理各种溶酶体贮积症的药物的商业领域,溶酶体贮积症是一种罕见的遗传病,其特征通常是由于酶缺乏导致溶酶体内未消化物质的积累。该市场包括一系列治疗产品,例如酶替代疗法、底物减少疗法和基因疗法,旨在解决 LSD 的各种临床表现,例如戈谢病、法布里病和庞贝病。随着认识的提高、研究和技术的进步以及管道疗法数量的不断增加,该市场有望大幅扩张。此外,医疗支出的增加和改善罕见病治疗可及性的举措进一步推动了市场增长,为制药公司和医疗保健提供商带来了机遇和挑战。
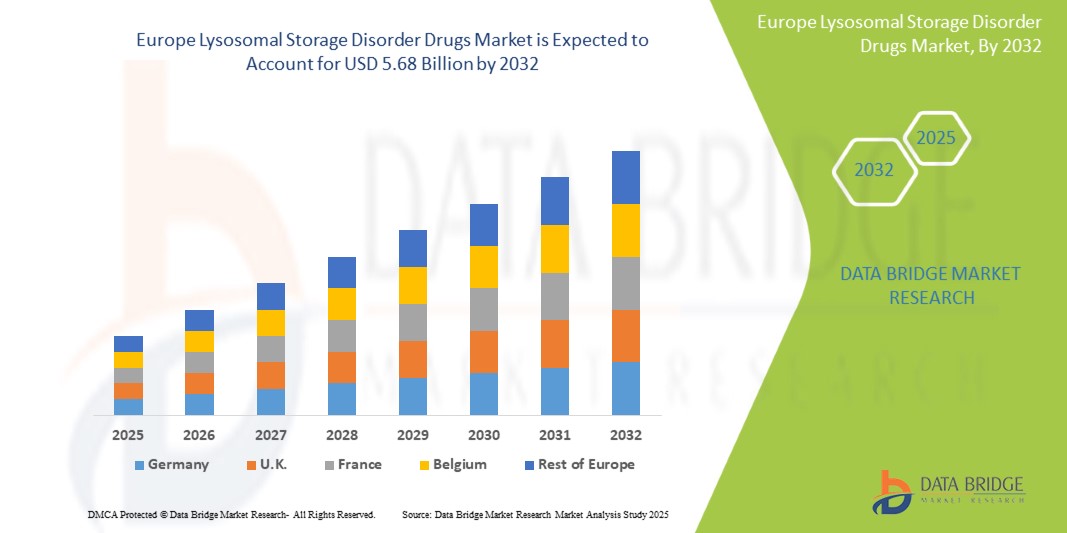
溶酶体储存症药物市场规模
2024 年欧洲溶酶体贮积症药物市场规模价值 29.2 亿美元,预计到 2032 年将达到 56.8 亿美元,在 2025 年至 2032 年的预测期内,复合年增长率为 8.7%。除了对市场价值、增长率、细分、地理覆盖范围和主要参与者等市场情景的见解外,Data Bridge Market Research 策划的市场报告还包括进出口分析、生产能力概览、生产消费分析、价格趋势分析、气候变化情景、供应链分析、价值链分析、原材料/消耗品概览、供应商选择标准、PESTLE 分析、波特分析和监管框架。
溶酶体储存障碍药物市场趋势
“溶酶体贮积症治疗的生物技术进展”
生物技术的进步在改变溶酶体贮积症 (LSD) 治疗前景方面发挥了关键作用,为患者提供了更有效、更个性化的治疗方案。酶替代疗法 (ERT) 通过直接解决导致这些疾病的酶缺陷,彻底改变了溶酶体贮积症 (LSD) 的治疗。在 LSD 患者中,特定酶的缺乏会导致细胞中积累毒性物质,损害器官和组织。ERT 通过使用合成酶来弥补缺失的酶,改善代谢功能并缓解症状。多年来,ERT 取得了长足的进步,新的、更有效的配方和给药方法可以改善酶的吸收并减少副作用。这些创新带来了更好的临床结果,这种趋势减缓了疾病进展,减少了器官损伤,提高了患者的生活质量。
报告范围和溶酶体储存障碍药物市场细分
|
属性 |
溶酶体储存障碍药物关键市场洞察 |
|
涵盖的领域 |
|
|
覆盖国家 |
德国、英国、意大利、法国、西班牙、俄罗斯、瑞士、土耳其、比利时、荷兰、丹麦、瑞典、波兰、挪威、芬兰和欧洲其他地区 |
|
主要市场参与者 |
赛诺菲(法国)、BioMarin(美国)、辉瑞公司(美国)、Amicus Therapeutics, Inc.(美国)、武田药品工业株式会社(日本)、Ultragenyx Pharmaceutical Inc.(美国)、Orchard Therapeutics plc(英国)、Spur Therapeutics(英国)、Sangamo Therapeutics(美国)、CHIESI Farmaceutici SpA(意大利)、REGENXBIO INC.(美国)和JCR Pharmaceuticals Co., Ltd.(日本)等 |
|
市场机会 |
|
|
增值数据信息集 |
除了对市场价值、增长率、细分、地理覆盖范围和主要参与者等市场情景的见解之外,Data Bridge Market Research 策划的市场报告还包括进出口分析、生产能力概览、生产消费分析、价格趋势分析、气候变化情景、供应链分析、价值链分析、原材料/消耗品概览、供应商选择标准、PESTLE 分析、波特分析和监管框架。 |
溶酶体储存症药物市场定义
溶酶体贮积症药物是指一类专门用于治疗溶酶体贮积症 (LSD) 的药物,LSD 是一组罕见的遗传疾病,由负责分解溶酶体内复杂分子的特定酶的缺陷引起。这些药物旨在通过替换缺失的酶(酶替代疗法)、增强人体产生酶的能力或修改由于酶缺乏而积累的底物(底物减少疗法)来解决与 LSD 相关的潜在代谢异常。通过改善酶功能或减少底物的毒性积累,这些治疗方法可以缓解症状、减缓疾病进展,并最终改善受这些棘手疾病影响的个人的生活质量。
溶酶体储存障碍药物市场动态
驱动程序
- 溶酶体贮积症的新兴个性化医疗
新兴的个性化医疗通过提供专门针对患者个人基因特征的疗法,正在改变溶酶体贮积症 (LSD) 治疗的格局。LSD 是由影响酶功能的基因突变引起的,这些突变在患者之间可能存在很大差异。个性化治疗涉及分析患者独特的基因组成,以制定更有针对性的方法,优化酶替代或基因治疗等疗法的疗效。这种精确性使治疗能够更好地匹配患者的特定需求,最大限度地减少副作用并提高治疗效果。随着基因检测和技术的进步,医疗保健提供者能够确定最合适的干预措施,从而有可能提高响应率和整体治疗成功率。向个性化医疗的转变正在加速定制 LSD 治疗的发展,这有望彻底改变疾病管理。随着这些创新,全球溶酶体贮积症药物市场对更有效、个性化治疗方案的需求不断增加。因此,个性化医疗是市场增长的主要驱动力,因为它可以提高治疗效果并改善患者结果,增强对治疗方案的信心。
例如,
2023 年 2 月,根据 NCBI 发布的文章,新兴的个性化医疗使用个人的基因图谱来指导与疾病预防、诊断和治疗相关的决策。这种方法可以更精确、有效地治疗溶酶体贮积症 (LSD),根据特定的基因突变量身定制治疗方案。随着个性化医疗变得越来越普遍,它推动了对有针对性的 LSD 疗法的需求,推动了全球溶酶体贮积症药物市场的增长。
- 政府增加对罕见病治疗研究的资助
政府对罕见病治疗研究的资助和补助对于推动溶酶体贮积症 (LSD) 等疾病的治疗方法的开发至关重要。罕见病由于患者人数少而往往缺乏商业可行性,给药物开发带来了重大挑战。为了解决这个问题,世界各国政府正在越来越多地拨出资金来激励这些医疗资源匮乏地区的研究。以补助、补贴和税收抵免形式提供的财政支持有助于制药公司和研究机构克服开发罕见病治疗方法的高成本和风险。此外,政府通常会加快这些治疗方法的监管程序,认识到迫切需要解决方案。通过减轻研究人员和公司的财务负担,这些资助机制使人们能够探索创新疗法,例如酶替代疗法和基因疗法,否则这些疗法将面临重大的开发障碍。
例如,
2023 年 8 月,根据美国国家罕见病组织发表的文章,美国国家罕见病组织宣布为罕见病研究提供超过 10 万美元的资助,凸显了政府对先进治疗的支持力度加大。这些资金使研究人员能够探索溶酶体贮积症 (LSD) 等罕见病的创新疗法。这种资金支持加速了药物开发,通过培育新的治疗选择推动了全球 LSD 药物市场的增长。
机会
- 正在研发的药物数量不断增加
溶酶体贮积症 (LSD) 药物研发管线数量不断增加,这为全球溶酶体贮积症药物市场带来了重大机遇,有望改善治疗方案并提高患者治疗效果。随着研究人员和制药公司对这些疾病的了解不断加深,他们正在开发各种新型疗法,包括基因疗法、底物减少疗法和小分子药物。这一扩大的研发管线反映了人们对 LSD 社区内未满足的医疗需求的日益认识,并有望为患者提供针对其具体情况的更广泛的治疗方式。创新疗法的引入可以提高患者对治疗的依从性,减轻疾病负担,并最终改善患有这些疾病的人的生活质量。
例如,
2024 年 6 月,Ultragenyx 宣布计划申请加速批准 UX111,用于治疗 A 型 Sanfilippo 综合征 (MPS IIIA)。UX111 是一种新型体内基因疗法,目前正处于 1/2/3 期开发阶段,用于治疗 A 型 Sanfilippo 综合征 (MPS IIIA),这是一种罕见的致命溶酶体贮积症,目前尚无获批的治疗方法,主要影响中枢神经系统。
- 越来越重视早期诊断
人们越来越重视溶酶体贮积症 (LSD) 的早期诊断,这为全球溶酶体贮积症药物市场提供了重大机遇,因为可以促进及时干预,从而大大改善患者的治疗效果。随着医疗保健系统通过改进的筛查计划、基因检测和诊断技术的进步更加重视早期检测,更多患者可能会在疾病的早期阶段得到诊断。这种早期干预不仅可以更好地控制症状,还可以提高现有和新兴疗法的潜在疗效。这种转变的结果是需要治疗方案并可以从中受益的患者群体更加广泛,最终推动溶酶体贮积症药物市场的需求。
例如,
2023 年 2 月,根据一篇题为“溶酶体贮积症:从生物学到临床,以印度为参考”的文章,早期诊断是 LSD 管理中最关键的部分,因为它为治疗干预、精准的遗传咨询、产前诊断和更好的患者结果提供了机会。
限制/挑战
- 医疗专业人士和患者缺乏意识
医疗专业人员和患者对溶酶体贮积症 (LSD) 缺乏认识,这对早期诊断、治疗和有效管理造成了重大障碍。许多医疗服务提供者,尤其是在罕见病接触有限的地区,往往无法识别 LSD 的症状,这些症状多种多样且与其他更常见的疾病重叠。这种知识的缺乏导致诊断延迟、误诊和治疗无效,加剧了疾病的严重性。对于患者,尤其是资源匮乏或服务不足地区的患者,缺乏认识会阻碍早期干预,使他们不知道潜在的治疗方法。LSD 的复杂性和罕见性使情况进一步复杂化,因为患者和医疗专业人员可能不完全了解早期治疗的重要性或专门护理选项的可用性。及时诊断和治疗的患者越来越少,导致临床结果不理想。这种认识的缺乏限制了对 LSD 特异性治疗的需求,并阻碍了全球溶酶体贮积症药物市场的整体增长。
例如,
2024 年 1 月,根据 Wiley 发表的文章,由于临床表型重叠和严重程度不同,成人 LSD 病例从症状出现到确诊通常需要大约 15 年的诊断延迟。这种延迟凸显了医疗保健专业人员(尤其是治疗成人患者的专业人员)缺乏意识。这种限制阻碍了早期发现和及时治疗,限制了全球溶酶体贮积症药物市场的增长。
- 药品审批程序冗长
冗长的药物审批流程对欧洲溶酶体贮积症药物市场造成了重大限制,减缓了急需的疗法的推出。获得新药监管批准的过程,尤其是针对 LSD 等罕见疾病,涉及大量临床试验、大量文献资料以及 FDA 和 EMA 等监管机构的严格评估。由于需要对安全性、有效性和潜在的长期影响进行全面评估,这些审批时间通常会延长。就 LSD 而言,这些疾病的罕见性和复杂性又增加了一层挑战,因为临床试验的患者人数有限,因此很难生成可靠的数据。这些罕见疾病需要专门的治疗,而缺乏既定的标准,这使审批过程变得复杂。因此,药物开发商面临漫长的等待期、新疗法上市延迟以及额外成本,这可能会让制药公司感到沮丧。因此,冗长的审批流程减缓了患者获得创新疗法的速度,降低了市场增长速度,阻碍了 LSD 有效治疗的可及性。这种监管延迟最终对全球溶酶体贮积症药物市场产生了重大限制。
例如,
2024年8月,根据Drugs.com发表的文章,药物的研究、开发和审批过程通常需要12到15年。这一延长的时间线推迟了新疗法的推出,并增加了制药公司的成本。因此,漫长的药物审批过程通过限制治疗的可用性来抑制全球LSD药物市场的增长。
本市场报告详细介绍了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响,分析了新兴收入领域的机会、市场法规的变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新。如需获取更多市场信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
溶酶体储存障碍药物市场范围
市场根据疾病类型、类型、药物、给药途径、年龄组、性别和分销渠道进行细分。这些细分市场之间的增长将帮助您分析行业中增长缓慢的细分市场,并为用户提供有价值的市场概览和市场洞察,帮助他们做出战略决策,确定核心市场应用。
疾病类型
- 戈谢病
- 类型 1
- 类型 3
- 类型 2
- 法布里病
- 庞贝病
- 婴儿型庞贝病
- 晚发型庞贝病
- 粘多糖贮积症(MPS)
- 粘多糖
- 骨髓增生异常综合征
- 粘多糖
- 甲基磺酸六号
- 骨髓增生异常综合征
- 尼曼匹克病
- C型
- B型
- A型
- 克拉伯病
- 其他的
类型
- 酶替代疗法 (ERT)
- 底物减量疗法 (SRT)
- 伴侣治疗
- 其他的
药物
- 伊米苷酶
- 阿加糖酶β
- 艾杜尔硫酸酶
- 阿尔法葡萄糖苷酶
- 维拉苷酶
- 塔利葡糖酶α
- 拉罗尼达酶
- 阿加糖酶α
- 加硫酸氢钾酶
- α-谷氨酰葡萄糖苷酶
- 其他的
给药途径
- 静脉注射 (IV)
- 皮下(SC)
- 口服
- 其他的
年龄组
- 儿科
- 成年人
- 老年
性别
- 男性
- 女性
分销渠道
- 医院药房
- 药店和零售药房
- 网上药店
溶酶体储存症药物市场区域分析
对市场进行分析,并根据上述疾病类型、类型、药物、给药途径、年龄组、性别和分销渠道提供市场规模洞察和趋势。
市场覆盖的国家包括美国、加拿大、墨西哥、德国、英国、意大利、法国、西班牙、俄罗斯、瑞士、土耳其、比利时、荷兰、丹麦、瑞典、波兰、挪威、芬兰和欧洲其他国家。
由于医疗保健基础设施的不断增加、溶酶体贮积症患病率的上升以及对先进疗法的认识不断提高,德国有望成为欧洲溶酶体贮积症药物市场中增长最快的国家。
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化会影响市场的当前和未来趋势。下游和上游价值链分析、技术趋势和波特五力分析、案例研究等数据点是用于预测单个国家市场情景的一些指标。此外,在提供国家数据的预测分析时,还考虑了欧洲品牌的存在和可用性以及它们因来自本地和国内品牌的激烈或稀缺竞争而面临的挑战、国内关税和贸易路线的影响。
溶酶体储存障碍药物市场份额
市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、欧洲业务、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度、应用主导地位。以上提供的数据点仅与公司对市场的关注有关。
溶酶体贮积症药物市场领导者有:
- 赛诺菲(法国)
- BioMarin (美国)
- 辉瑞公司 (美国)
- Amicus Therapeutics, Inc.(美国)
- 武田药品工业株式会社 (日本)
- Ultragenyx Pharmaceutical Inc.(美国)
- Orchard Therapeutics plc(英国)
- Spur Therapeutics(英国)
- Sangamo Therapeutics(美国)
- CHIESI Farmaceutici SpA(意大利)
- REGENXBIO INC.(美国)
- JCR制药株式会社(日本)
溶酶体贮积症药物市场的最新发展
- 2024 年 10 月,Amicus Therapeutics 宣布就其治疗法布里病的药物 Galafold (migalastat) 达成和解。该和解解决了正在进行的专利纠纷,并确认该药物的营销将继续进行,不受诉讼干扰。这项与多方达成的协议旨在为 Galafold 的可用性和开发提供稳定性,同时保护知识产权
- 2024 年 6 月,Amicus Therapeutics 因其用于治疗法布里病的创新疗法 Pombiliti (miglustat) 荣获 Prix Galien UK 奖。该奖项表彰了制药创新方面的卓越成就,并强调了 Pombiliti 在改善罕见遗传病患者生活方面的影响。这一荣誉凸显了 Amicus 在罕见病治疗领域的领导地位
- 2024 年 4 月,Forge Biologics 宣布将在 2024 年 5 月举行的 ASGCT 第 27 届年会上发表 9 场演讲,包括一场最新口头演讲和三场技术会议。演讲将涵盖工艺开发、分子进展和临床更新,包括 FBX-101 在克拉伯病中的重大临床结果
- 2023 年 11 月,Chiesi Group 已在意大利、澳大利亚、美国等多个地区重新获得卓越职场认证。Chiesi 的员工回复率为 85%,总体满意率为 83%,这反映了其致力于营造积极、包容、协作的工作环境,注重员工的福祉和成长
- 2024 年 1 月,生物制药公司 Denali Therapeutics Inc. 宣布了 2024 年的进展和里程碑,该公司正在开发一种能够跨越血脑屏障治疗神经退行性疾病和溶酶体贮积症的疗法。首席执行官 Ryan Watts 博士在 1 月 9 日举行的第 42 届摩根大通年度医疗保健大会上的公司演讲中重点介绍了这些进展
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PORTER’S FIVE FORCES
4.2 PIPELINE ANALYSIS
5 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN EUROPE
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDERS TREATMENTS
6.1.2 EMERGING PERSONALIZED MEDICINE FOR LYSOSOMAL STORAGE DISORDERS
6.1.3 INCREASED GOVERNMENT FUNDING FOR RESEARCH INTO RARE DISEASE TREATMENTS
6.1.4 COLLABORATIONS AND PARTNERSHIPS BETWEEN PHARMACEUTICAL COMPANIES AND RESEARCH INSTITUTIONS
6.2 RESTRAINTS
6.2.1 LACK OF AWARENESS AMONG HEALTHCARE PROFESSIONALS AND PATIENTS
6.2.2 PROLONGED DRUG APPROVAL PROCEDURES
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE DRUGS
6.3.2 GROWING EMPHASIS ON EARLY DIAGNOSIS
6.3.3 ADVANCEMENTS IN GENE THERAPY FOR THE TREATMENT OF LYSOSOMAL STORAGE DISORDERS
6.4 CHALLENGES
6.4.1 SIGNIFICANT EXPENSES RELATED TO THE TREATMENT OF THE DISEASE
6.4.2 NARROW PATIENT BASE SUFFERING FROM LYSOSOMAL STORAGE DISORDERS
7 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
7.1 OVERVIEW
7.2 GAUCHER DISEASE
7.2.1 TYPE 1
7.2.2 TYPE 3
7.2.3 TYPE 2
7.3 FABRY DISEASE
7.4 POMPE DISEASE
7.4.1 INFANTILE-ONSET POMPE
7.4.2 LATE-ONSET POMPE
7.5 MUCOPOLYSACCHARIDOSIS (MPS)
7.5.1 MPS I
7.5.2 MPS II
7.5.3 MPS IV
7.5.4 MPS VI
7.5.5 MPS III
7.6 NIEMANN-PICK DISEASE
7.6.1 TYPE C
7.6.2 TYPE B
7.6.3 TYPE A
7.7 KRABBE DISEASE
7.8 OTHERS
8 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS
8.1 OVERVIEW
8.2 IMIGLUCERASE
8.3 AGALSIDASE BETA
8.4 IDURSULFASE
8.5 ALGLUCOSIDASE ALPHA
8.6 VELAGLUCERASE
8.7 TALIGLUCERASE ALFA
8.8 LARONIDASE
8.9 AGALSIDASE ALPHA
8.1 GALSULFASE
8.11 AVALGLUCOSIDASE ALFA
8.12 OTHERS
9 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE
9.1 OVERVIEW
9.2 ENZYME REPLACEMENT THERAPY (ERT)
9.3 SUBSTRATE REDUCTION THERAPY (SRT)
9.4 CHAPERONE THERAPY
9.5 OTHERS
10 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER
10.1 OVERVIEW
10.2 MALE
10.3 FEMALE
11 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
11.4 GERIATRIC
12 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 HOSPITAL PHARMACIES
12.3 DRUGS STORES AND RETAIL PHARMACIES
12.4 ONLINE PHARMACIES
13 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 INTRAVENOUS (IV)
13.3 SUBCUTANEOUS (SC)
13.4 ORAL
13.5 OTHERS
14 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 UNITED KINGDOM
14.1.3 FRANCE
14.1.4 ITALY
14.1.5 SPAIN
14.1.6 RUSSIA
14.1.7 SWITZERLAND
14.1.8 TURKEY
14.1.9 BELGIUM
14.1.10 NETHERLANDS
14.1.11 DENMARK
14.1.12 SWEDEN
14.1.13 POLAND
14.1.14 NORWAY
14.1.15 FINLAND
14.1.16 REST OF EUROPE
15 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILES
17.1 SANOFI
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 BIOMARIN
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 PFIZER INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 TAKEDA PHARMACEUTICAL COMPANY LIMITED
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 AMICUS THERAPEUTIC, INC.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CHIESI FARMACEUTICI S.P.A.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 DENALI THERAPEUTICS
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PIPELINE PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 FORGE BIOLOGICS
17.8.1 COMPANY SNAPSHOT
17.8.2 PIPELINE PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.9 JCR PHARMACEUTICALS CO., LTD.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PINELINE PRODUCT PORTFOLIO
17.9.4 PRODUCT PORTFOLIO
17.9.5 RECENT DEVELOPMENT
17.1 ORCHARD THERAPEUTICS PLC
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 PROTALIX BIOTHERAPEUTICS
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PIPELINE PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.12 REGENXBIO INC.
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PINELINE PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENT
17.13 SANGAMO THERAPEUTICS
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 SPUR THERAPEUTICS
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 ULTRAGENYX PHARMACEUTICAL INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
表格列表
TABLE 1 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 2 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 3 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 4 EUROPE FABRY DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 5 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 6 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 7 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 8 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 9 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 10 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 11 EUROPE KRABBE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 12 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 13 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 14 EUROPE IMIGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 15 EUROPE AGALSIDASE BETA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 16 EUROPE IDURSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 17 EUROPE ALGLUCOSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 18 EUROPE VELAGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 19 EUROPE TALIGLUCERASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 20 EUROPE LARONIDASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 21 EUROPE AGALSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 22 EUROPE GALSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 23 EUROPE AVALGLUCOSIDASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 24 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 25 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 26 EUROPE ENZYME REPLACEMENT THERAPY (ERT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 27 EUROPE SUBSTRATE REDUCTION THERAPY (SRT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 28 EUROPE CHAPERONE THERAPY IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 29 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 30 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 31 EUROPE MALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 32 EUROPE FEMALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 33 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 34 EUROPE PEDIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 35 EUROPE ADULT IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 36 EUROPE GERIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 37 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 38 EUROPE HOSPITAL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 39 EUROPE DRUGS STORES AND RETAIL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 40 EUROPE ONLINE PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 41 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 42 EUROPE INTRAVENOUS (IV) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 43 EUROPE SUBCUTANEOUS (SC) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 44 EUROPE ORAL IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 45 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 46 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 47 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 48 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 49 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 50 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 51 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 52 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 53 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 54 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 55 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 56 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 57 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 58 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 59 GERMANY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 60 GERMANY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 61 GERMANY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 62 GERMANY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 63 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 64 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 65 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 66 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 67 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 68 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 69 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 70 UNITED KINGDOM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 71 UNITED KINGDOM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 72 UNITED KINGDOM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 73 UNITED KINGDOM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 74 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 75 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 76 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 77 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 78 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 79 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 80 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 81 FRANCE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 82 FRANCE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 83 FRANCE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 84 FRANCE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 85 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 86 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 87 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 88 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 89 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 90 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 91 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 92 ITALY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 93 ITALY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 94 ITALY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 95 ITALY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 96 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 97 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 98 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 99 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 100 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 101 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 102 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 103 SPAIN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 104 SPAIN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 105 SPAIN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 106 SPAIN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 107 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 108 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 109 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 110 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 111 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 112 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 113 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 114 RUSSIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 115 RUSSIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 116 RUSSIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 117 RUSSIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 118 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 119 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 120 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 121 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 122 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 123 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 124 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 125 SWITZERLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 126 SWITZERLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 127 SWITZERLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 128 SWITZERLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 129 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 130 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 131 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 132 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 133 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 134 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 135 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 136 TURKEY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 137 TURKEY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 138 TURKEY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 139 TURKEY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 140 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 141 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 142 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 143 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 144 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 145 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 146 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 147 BELGIUM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 148 BELGIUM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 149 BELGIUM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 150 BELGIUM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 151 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 152 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 153 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 154 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 155 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 156 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 157 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 158 NETHERLANDS GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 159 NETHERLANDS POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 160 NETHERLANDS MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 161 NETHERLANDS NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 162 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 163 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 164 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 165 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 166 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 167 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 168 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 169 DENMARK GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 170 DENMARK POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 171 DENMARK MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 172 DENMARK NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 173 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 174 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 175 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 176 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 177 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 178 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 179 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 180 SWEDEN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 181 SWEDEN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 182 SWEDEN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 183 SWEDEN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 184 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 185 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 186 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 187 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 188 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 189 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 190 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 191 POLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 192 POLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 193 POLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 194 POLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 195 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 196 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 197 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 198 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 199 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 200 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 201 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 202 NORWAY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 203 NORWAY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 204 NORWAY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 205 NORWAY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 206 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 207 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 208 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 209 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 210 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 211 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 212 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 213 FINLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 214 FINLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 215 FINLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 216 FINLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 217 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 218 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 219 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 220 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 221 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 222 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 223 REST OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
图片列表
FIGURE 1 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 2 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 SEVEN SEGMENTS COMPRISE THE EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDER TREATMENTS IS EXPECTED TO DRIVE THE EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 14 GAUCHER DISEASE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN 2025 & 2032
FIGURE 15 PESTEL ANALYSIS
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET
FIGURE 17 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2024
FIGURE 18 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2025-2032 (USD MILLION)
FIGURE 19 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, CAGR (2025-2032)
FIGURE 20 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, LIFELINE CURVE
FIGURE 21 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2024
FIGURE 22 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2025-2032 (USD MILLION)
FIGURE 23 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, CAGR (2025-2032)
FIGURE 24 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, LIFELINE CURVE
FIGURE 25 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2024
FIGURE 26 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2025-2032 (USD MILLION)
FIGURE 27 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 28 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 29 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2024
FIGURE 30 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2025-2032 (USD MILLION)
FIGURE 31 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, CAGR (2025-2032)
FIGURE 32 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 33 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2024
FIGURE 34 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2025-2032 (USD MILLION)
FIGURE 35 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, CAGR (2025-2032)
FIGURE 36 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 37 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 38 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD MILLION)
FIGURE 39 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 40 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2024
FIGURE 42 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2025-2032 (USD MILLION)
FIGURE 43 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2025-2032)
FIGURE 44 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 45 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SNAPSHOT 2024
FIGURE 46 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。