Global Anal Cancer Drug Market
市场规模(十亿美元)
CAGR :
% 
 USD
401.57 Million
USD
1,199.82 Million
2024
2032
USD
401.57 Million
USD
1,199.82 Million
2024
2032
| 2025 –2032 | |
| USD 401.57 Million | |
| USD 1,199.82 Million | |
|
|
|
|
全球肛門癌藥物市場細分,按類型(鱗狀細胞癌、腺癌、基底細胞癌、黑色素瘤和小細胞癌)、治療方法(藥物和手術)、藥物(Gradasil、氟尿嘧啶、絲裂黴素、順鉑等)、給藥途徑(口服和腸外)、分銷管道(醫院藥房、零售藥房和網上藥房)、最終
肛門癌藥物市場分析
近年來,隨著人們對肛門癌認識的提高、治療方案的進步以及對該疾病的了解的加深,肛門癌藥物市場出現了顯著增長。肛門癌雖然罕見,但每年影響數千人,這促使對有效治療方法的需求增加。化療、放射療法和手術幹預等傳統治療方法已得到更新的標靶療法的補充,旨在改善治療效果並減少副作用。例如,歐盟委員會批准Opdivo(nivolumab)和Yervoy(ipilimumab)用於治療肛門癌患者,這是一個重要的里程碑。這些免疫療法代表了治療方法的一個有希望的轉變,為患者提供了傳統療法的替代方法。此外,抗EGFR和抗VEGF療法的引入進一步擴大了治療前景。隨著精準醫療的不斷進步,隨著更有針對性的治療方法的開發,市場預計將進一步成長。政府和醫療機構也越來越多地支持研究和治療機會,為肛門癌藥物市場帶來更樂觀的前景。
肛門癌藥物市場規模
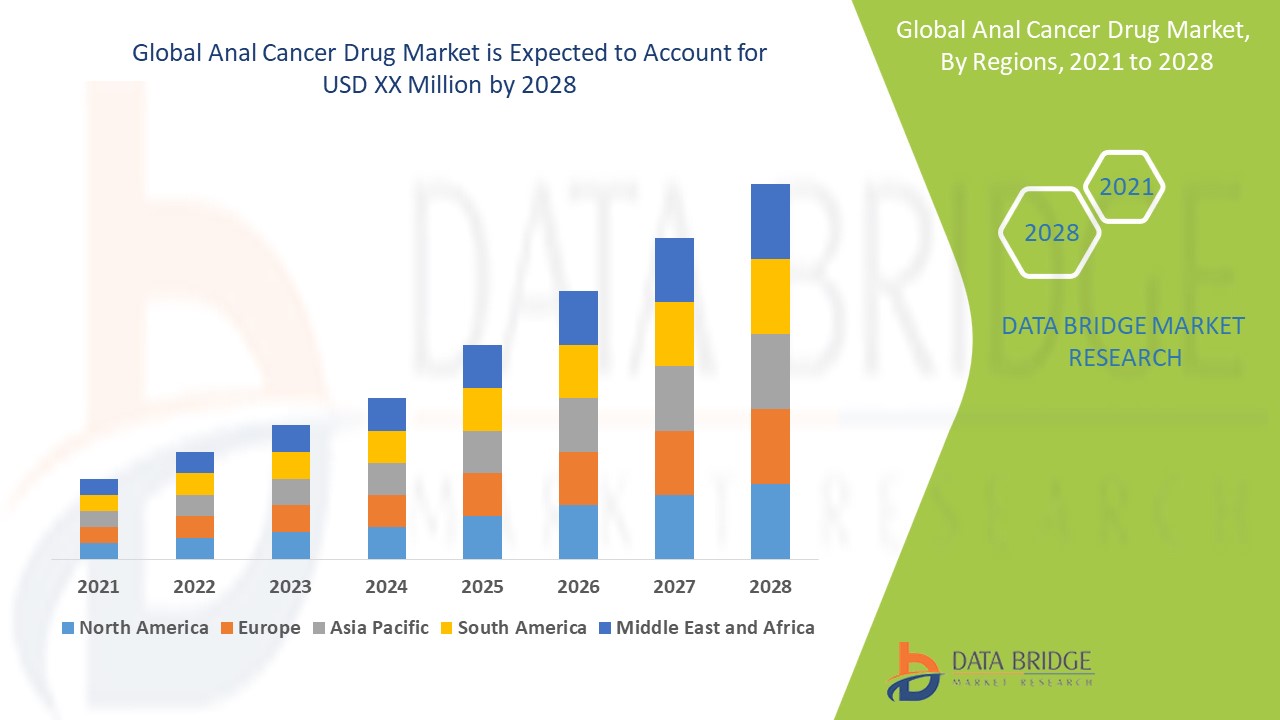
2024 年全球肛門癌藥物市場規模為 4.0157 億美元,預計到 2032 年將達到 11.9982 億美元,在 2025 年至 2032 年的預測期內複合年增長率為 13.80%。除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。
肛門癌藥物市場趨勢
“免疫療法作為一線治療方案的應用日益廣泛”
肛門癌藥物市場的關鍵趨勢是越來越多地使用免疫療法作為第一線治療選擇。 Opdivo(nivolumab)和Yervoy(ipilimumab)等免疫療法藥物在治療肛門癌方面已顯示出良好的效果,尤其是在轉移性或晚期病例中。這些療法透過刺激患者的免疫系統來瞄準和摧毀癌細胞,比傳統療法更有效。例如,Opdivo 和 Yervoy 已獲得歐盟委員會批准用於治療肛門癌,這標誌著治療方法的重大轉變。越來越多的臨床證據和聯合療法研究進一步支持了這一趨勢,例如將 nivolumab 與化療結合以增強治療效果。隨著免疫療法的應用不斷增長,肛門癌藥物市場預計將擴大,這得益於人們日益轉向更有針對性的個人化治療,以最大限度地減少副作用並提高患者的生存率。
報告範圍和肛門癌藥物市場細分
|
屬性 |
肛門癌藥物關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
北美洲的美國、加拿大和墨西哥、德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其、歐洲其他地區、中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區 (APAC) 的其他地區、沙烏地阿拉伯、阿聯酋、南非、埃及、以色列、中東和非洲 (MEA) 的其他地區、其他地區的歐洲地區 |
|
主要市場參與者 |
Ayala Pharmaceuticals(美國)、台灣脂質體股份有限公司(台灣)、INOVIO Pharmaceuticals, Inc(美國)、F. Hoffmann-La Roche Ltd(瑞士)、GSK plc(英國)、Merck & Co., Inc(美國)、Amgen Inc(美國)、AntOn Biosciences, Inc.(美國)、Yeuptics(愛爾蘭)、Yaccon) & Co. KG(德國)、The Emmes Company, LLC(美國)、BioMimetix(美國)、QIAGEN(德國)和 Castle Biosciences, Inc(美國) |
|
市場機會 |
|
|
加值資料資訊集 |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Anal Cancer Drug Market Definition
Anal cancer drugs are pharmaceutical treatments specifically designed to treat anal cancer, a rare form of cancer that affects the tissues of the anus. These drugs include chemotherapy, radiation therapy, immunotherapies, and targeted therapies. Chemotherapy drugs, such as fluorouracil and cisplatin, work by killing rapidly dividing cancer cells, while radiation therapy uses high-energy rays to target and destroy cancerous cells.
Anal Cancer Drug Market Dynamics
Drivers
- Increasing Incidence of Anal Cancer
The increasing incidence of anal cancer is a major driver in the anal cancer drug market, as the number of cases continues to rise, particularly among high-risk populations. For instance, studies show that individuals with human papillomavirus (HPV) infections, especially HPV types 16 and 18, are at significantly higher risk for developing anal cancer. The Centers for Disease Control and Prevention (CDC) reports that men who have sex with men (MSM), particularly those who are HIV-positive, are at a much higher risk for anal cancer, with rates up to 35 times higher compared to the general population. This growing prevalence, coupled with the aging population and an increasing number of immunocompromised individuals, is driving the demand for more effective treatment options. The rise in anal cancer cases, especially within these vulnerable groups, is accelerating the need for new therapies, making it a key driver for the anal cancer drug market. As awareness and early diagnosis improve, the market is expected to expand significantly.
- Increasing Awareness and Advancements in Diagnostic Tools
Increasing awareness and advancements in diagnostic tools are creating a significant market opportunity in the anal cancer drug market by enabling earlier detection and improving treatment outcomes. With rising awareness about the risk factors and symptoms of anal cancer, more people are seeking screening, leading to earlier diagnoses. The availability of improved diagnostic methods, such as HPV DNA testing and high-resolution anoscopy (HRA), has also enhanced the ability to detect anal cancer at its precancerous stages, allowing for more. effective intervention. For instance, the adoption of HRA in clinical practice has led to earlier identification of high-risk lesions in patients with HIV or HPV, ultimately improving survival rates. This shift toward early detection is increasing the demand for specialized drugs, particularly those that target specific stages of the disease or offer personalized treatment. As a result, the growing emphasis on early screening and diagnosis presents a significant market opportunity, further driving innovation and development in the anal cancer treatment landscape.
Opportunities
- Increasing Advancements in Treatment Options
Advancements in treatment options are creating a substantial market opportunity in the anal cancer drug market, particularly with the approval and increasing use of immunotherapies and targeted therapies. Immunotherapies such as nivolumab (Opdivo) and ipilimumab (Yervoy) have revolutionized the treatment of anal cancer, offering new hope, especially for patients with advanced or metastatic disease. For instance, nivolumab, an immune checkpoint inhibitor, has shown promising results in clinical trials when combined with other treatments, improving survival rates and reducing tumor progression. In addition, the rise of targeted therapies, which focus on specific molecular targets within cancer cells, is broadening the treatment landscape. These therapies aim to minimize side effects while enhancing therapeutic efficacy. The approval of Opdivo and Yervoy by the European Commission for the treatment of anal cancer patients reflects a growing trend toward precision medicine, making these advancements a key driver of growth in the market. As more therapies are developed and integrated into treatment regimens, the expansion of treatment options will further stimulate the demand for specialized drugs, presenting a clear market opportunity.
- Increasing Government Support and Research Initiatives
隨著對癌症研究的投資增加和新療法的開發繼續加速成長,政府的支持和研究措施正在推動肛門癌藥物市場的巨大市場機會。世界各國政府都在資助癌症研究計畫並提供補助金來支持創新治療方法的發展。例如,在美國,國家癌症研究所(NCI)撥出大量資金用於肛門癌研究,特別注重免疫療法和標靶治療等新療法。此外,FDA 的孤兒藥指定和快速通道計畫等政府支持的措施有助於加快新藥的審批流程,進一步鼓勵有前景的療法進入市場。一個例子是 FDA 批准 nivolumab(Opdivo)和 ipilimumab(Yervoy)用於治療肛門癌,這得到了政府支持的臨床試驗和研究的支持。這項資金和簡化的審批流程正在擴大治療選擇並為新療法創造沃土,最終為參與開發和行銷肛門癌藥物的公司提供市場機會。
限制/挑戰
- 治療費用高昂
高昂的治療費用是肛門癌藥物領域的重大市場挑戰,因為它給許多患者帶來了經濟障礙並限制了他們獲得治療的機會。例如,較新的免疫療法和標靶生物藥物在治療各種癌症方面顯示出良好的前景,但價格卻非常昂貴,為已經很昂貴的化療和放療組合增加了相當大的成本。高昂的治療費用會對患者和醫療保健系統造成沉重的負擔,尤其是在保險覆蓋有限或醫療保健不足的地區。例如,患者可能面臨購買基本藥物的高額自付費用,或者可能由於經濟壓力而被迫推遲或放棄治療。此外,許多保險公司可能只為某些治療提供有限的保險,這進一步加劇了這個問題。這個成本問題阻礙了個人獲得可能挽救生命的治療方法,並限制了新治療方法的整體成長和市場滲透,對有效肛門癌治療方法的開發和廣泛採用構成了重大挑戰。
- 肛門癌的恥辱
肛門癌的恥辱感及其嚴重的心理影響代表著一項重大的市場挑戰,因為它阻礙了許多患者及時就醫和堅持治療。由於疾病會影響肛門區域的敏感性,患者常常感到尷尬或羞愧,這會導致診斷延遲。例如,許多人最初可能會將肛門出血或不適等症狀視為痔瘡,避免與醫療保健提供者討論,直到病情惡化。診斷結果給患者帶來的情緒壓力加劇了患者尋求幫助的猶豫,使他們感到焦慮、憂鬱,自尊心受到打擊。與肛門癌相關的心理困擾也會影響患者接受化療和放療等侵入性治療的意願,可能導致治療順從性降低和治療效果較差。圍繞這種疾病的恥辱感限制了人們的認識,並使患者更難公開討論治療方案,從而使市場更加複雜。這給患者參與和標靶治療的開發帶來了障礙,因為與其他癌症相比,該市場規模較小且不太引人注目。
本市場報告提供了最新發展、貿易法規、進出口分析、生產分析、價值鏈優化、市場份額、國內和本地化市場參與者的影響的詳細信息,分析了新興收入領域的機會、市場法規的變化、戰略市場增長分析、市場規模、類別市場增長、應用領域和主導地位、產品批准、產品發布、地理擴展、市場技術創新。要獲取更多市場信息,請聯繫 Data Bridge Market Research 獲取分析師簡報,我們的團隊將幫助您做出明智的市場決策,實現市場成長。
肛門癌藥物市場範圍
市場根據類型、治療、藥物、給藥途徑、分銷管道和最終用戶進行細分。這些細分市場之間的成長將幫助您分析行業中微弱的成長細分市場,並為用戶提供有價值的市場概覽和市場洞察,幫助他們做出策略決策,確定核心市場應用。
類型
- 鱗狀細胞癌
- 腺癌
- 基底細胞癌
- 黑色素瘤
- 小細胞癌
治療
- 藥物
- 手術
藥物
- 格拉達西爾
- 氟尿嘧啶
- 絲裂黴素
- 順鉑
- 其他的
給藥途徑
- 口服
- 腸外
分銷管道
- 醫院藥房
- 零售藥局
- 網路藥局
最終用戶
- 醫院
- 居家護理
- 專科診所
- 其他的
肛門癌藥物市場區域分析
對市場進行分析,並按國家、類型、治療、藥物、給藥途徑、分銷管道和最終用戶提供市場規模洞察和趨勢,如上所述。
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the anal cancer drug market, driven by increased awareness among consumers regarding the diagnosis and treatment of colorectal cancer. In addition, rising government support for the advancement of colorectal cancer surgeries and drug development is expected to further accelerate the growth of the anal cancer drug market in the region throughout the forecast period.
Asia-Pacific is forecasted to be the fastest-growing market in the global anal cancer drug sector. This growth is attributed to the increasing incidence of anal cancer, improved healthcare access, and rising awareness about early diagnosis and treatment. In addition, the expansion of healthcare infrastructure and government initiatives to address cancer treatment are expected to boost market development. As a result, the region is anticipated to experience rapid growth in the anal cancer drug market in the coming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Anal Cancer Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Anal Cancer Drug Market Leaders Operating in the Market Are:
- Ayala Pharmaceuticals (U.S.)
- TAIWAN LIPOSOME CO., LTD. (Taiwan)
- INOVIO Pharmaceuticals, Inc (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- GSK plc (U.K.)
- Merck & Co., Inc (U.S.)
- Amgen Inc (U.S.)
- Antiva Biosciences, Inc (U.S.)
- Medtronic (Ireland)
- Xencor (U.S.)
- Onconova Therapeutics (U.S.)
- ORYX GmbH & Co. KG (Germany)
- The Emmes Company, LLC (U.S.)
- BioMimetix (U.S.)
- QIAGEN (Germany)
- Castle Biosciences, Inc (U.S.)
Latest Developments in Anal Cancer Drug Market
- In June 2024, Merck announced significant advancements in its cancer drug development pipeline, including Phase II trials for tuvusertib (an ATR inhibitor) and M9466 (a PARP1 inhibitor). In addition, Merck progressed M9140 (anti-CEACAM5 ADC) and M3554 (novel anti-GD2 ADC) into clinical stages
- In June 2024, Eli Lilly reported positive results from an early-stage trial of olomorasib, a second-generation KRAS G12C inhibitor, which demonstrated efficacy against various solid tumors with KRAS G12C mutations, including non-small cell lung cancer. In this trial, olomorasib was combined with KEYTRUDA (pembrolizumab), a PD-1 inhibitor from Merck, which showed good tolerability when used together in another study
- In August 2023, Taiho Oncology, Inc. and Taiho Pharmaceutical Co., Ltd. announced the FDA approval of LONSURF® (trifluridine/tipiracil) for the treatment of adults with metastatic colorectal cancer (mCRC). In addition, the approval followed prior treatments with anti-VEGF biological therapy, fluoropyrimidine, oxaliplatin, or irinotecan-based chemotherapy, and, for RAS wild-type patients, an anti-EGFR therapy
- In June 2023, AstraZeneca and Daiichi Sankyo revealed positive results from the DESTINY CRC02 phase trial for Enhertu, showcasing both efficacy and safety in patients with HER-2 overexpressing metastatic colorectal cancer who had previously undergone treatment
- In June 2021, Bristol Myers Squibb received European Commission approval for the combination of Opdivo and Yervoy to treat anal cancer patients, granting marketing authorizations across Europe
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。