全球聽力學設備市場,依產品(骨錨式助聽器、人工耳蝸、助聽器、診斷設備、鼓室計、聽力計、耳鏡)、類型(RITE(耳內接收器)助聽器、ITE(耳內)助聽器、BTE(耳背)助聽器、耳道式助聽器)、技術(數位、類比)、銷售管道(零售、政府採購、電子商務)、疾病類型(耳硬化症、梅尼爾氏症、聽神經腫瘤、中耳炎等)、最終用戶(醫院、門診手術中心(ASC)、研究機構)劃分-產業趨勢及 2029 年預測。
市場分析與規模
聽力設備市場在很大程度上受到製造商日益重視開發融合尖端技術的產品,並與其他機構合作以促進其產品更快的推廣和宣傳的影響。此外,由於遠端設備的優勢以及日益成熟的人群的認可,人們對其日益增長的偏好預計將在預測期內對市場的成長產生積極影響。
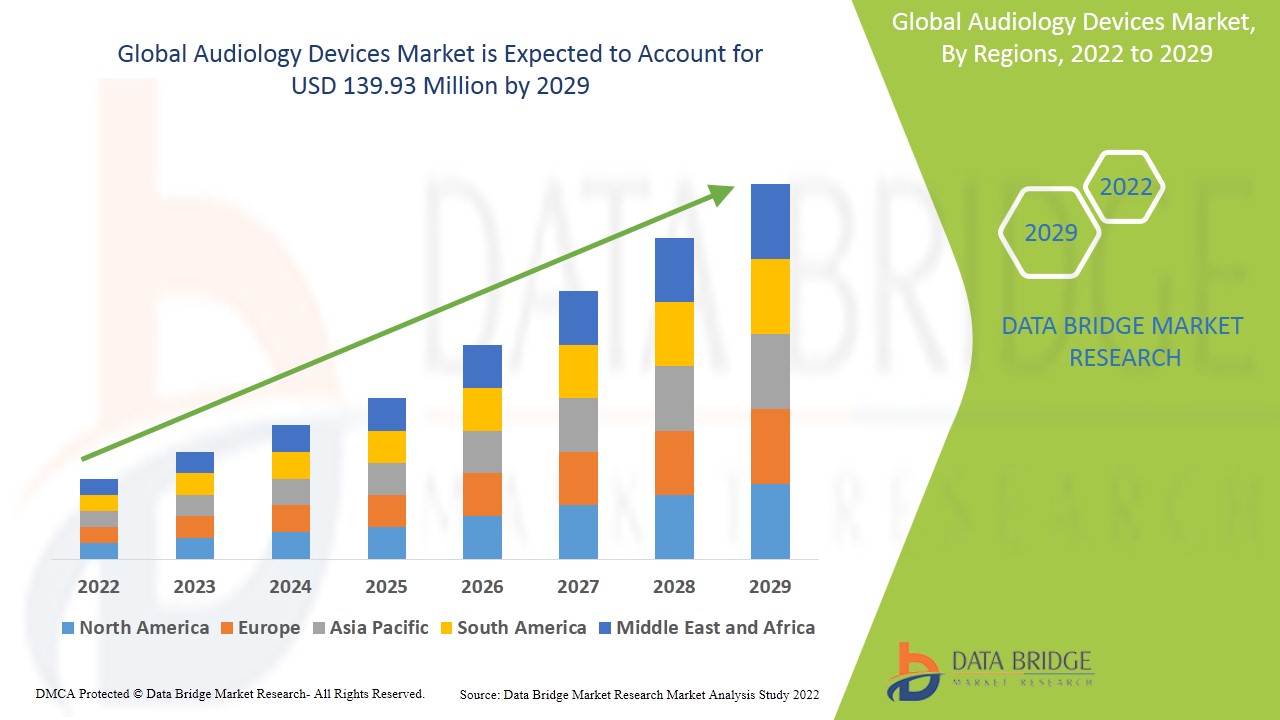
- Data Bridge Market Research 分析稱,預計到 2029 年,聽力設備市場規模將達到 1.3993 億美元,預測期內複合年增長率為 5.45%。 「骨錨式助聽器」是聽力設備市場中最大的產品類別,預計在 2022 年至 2029 年的預測期內,其複合年增長率將達到 6.5%。
市場定義
聽力學設備是聽力學家用來診斷和治療聽力損失和聽力障礙的電子設備。這些設備也可用於測試或監測聽力。這些設備的尺寸、形狀、功能和聽力狀況各不相同。聽力學設備既可用於診斷,也可用於治療聽力障礙。
聽力學設備市場動態
驅動程式
-
聽力問題盛行率高
人群中聽力損傷和聽力損失的發生率不斷上升,尤其是在老齡化人口中,是推動該市場成長的最重要因素。全球人口的成長也導致聽力問題患者比例上升,預計這將加速市場的整體成長。
技術進步、經濟高效的設備(例如無線設備)以及老年人群對新型設備的廣泛接受,預計也將推動市場成長。此外,政府為方便用戶取得助聽器而推出的支持性舉措,也有助於預測期內市場的成長。
機會
此外,改進的診斷性能和治療決策(例如基於 PC 的聽力計和混合聽力計的進步)可以輕鬆地與標準計算機和操作系統集成,這為 2022 年至 2029 年預測期內的市場參與者帶來了盈利機會。此外,積極的監管環境將進一步擴大聽力設備市場未來的成長率。
限制/挑戰
另一方面,與耳聾相關的社會恥辱預計將阻礙市場成長。此外,聽力障礙解決方案(尤其是基於手術的解決方案)的高昂價格預計將在2022-2029年的預測期內對聽力學設備市場構成挑戰。
本聽力學設備市場報告詳細介紹了最新發展動態、貿易法規、進出口分析、生產分析、價值鏈優化、市場份額、國內和本地市場參與者的影響,並分析了新興收入來源、市場法規變化、戰略市場增長分析、市場規模、品類市場增長、應用領域和市場主導地位、產品審批、產品發布、地域擴張以及市場技術創新等方面的機遇。如需了解更多關於聽力學設備市場的信息,請聯繫 Data Bridge 市場研究部門獲取分析師簡報,我們的團隊將幫助您做出明智的市場決策,實現市場成長。
COVID-19對聽力學設備市場的影響
新冠疫情對市場產生了負面影響。疫情為失聰或聽力障礙的住院病患帶來了額外的挑戰。此外,聽力設備的需求呈現下降趨勢。治療方面的限制也限制了市場的成長。然而,新冠疫情加速了全球遠距醫療的普及。許多醫療保健專業人員正在轉向遠距聽力學,以避免患者人流擁擠,預計這將為市場創造有利環境。
最新動態
- 2020 年 7 月,人工耳蝸 Nucleus Kanso 2 聲音處理器、適用於 Nucleus 22 植入者的 Nucleus 7 聲音處理器以及 Custom Sound Pro 驗配軟體均已獲得美國 FDA 批准。
- 2021 年 2 月,GN Hearing(丹麥)推出了助聽器系列 ReSound Key,擴大了該公司久經考驗且屢獲殊榮的聽力技術的全球使用範圍。
- 2020年10月,美國Starkey公司與以色列OrCam Technologies公司合作,為聽力和視力障礙者提供輔助技術。透過與Starkey的Livio Edge AI助聽器合作,OrCam能夠利用其開發的先進電腦視覺和機器學習方法,透過音訊傳遞視覺世界。
全球聽力設備市場範圍
聽力學設備市場根據產品、類型、技術、銷售管道、疾病類型和最終用戶進行細分。這些細分市場的成長將有助於您分析行業中成長乏力的細分市場,並為用戶提供有價值的市場概覽和市場洞察,幫助他們做出策略決策,確定核心市場應用。
產品
- 骨錨式助聽器
- 人工耳蝸
- 助聽器
- 診斷設備
- 鼓室壓力計
- 聽力計
- 耳鏡
根據產品類型,聽力學設備市場可細分為骨錨式助聽器、人工耳蝸、助聽器、診斷設備、鼓室計、聽力計和耳鏡。預計骨錨式助聽器將在該細分市場中實現最高成長。
類型
- RITE(耳內接收器)助聽器
- ITE(耳內式)助聽器
- 耳背式助聽器
- 耳道式助聽器
根據類型,聽力學設備市場細分為耳內式助聽器 (RITE)、耳內式助聽器 (ITE)、耳背式助聽器 (BTE) 和耳道式助聽器。耳道式助聽器又細分為耳道式助聽器 (ITC)、耳道式助聽器 (CIC) 和隱形耳道式助聽器 (IIC)。
科技
- 數位的
- 類似物
根據技術,聽力設備市場分為數位和類比兩大類。數位助聽器憑藉其快速的適應性,預計將在技術領域佔據主導地位。
銷售通路
- 零售銷售
- 政府採購
- 電子商務
根據銷售管道級別,聽力設備市場分為零售、政府採購和電子商務。
疾病類型
- 耳硬化症
- 梅尼爾氏症
- 聽覺腫瘤
- 中耳炎
- 其他的
依疾病類型,聽力設備市場分為耳硬化症、梅尼爾氏症、聽覺腫瘤、中耳炎等
終端用戶
- 醫院
- 門診手術中心(ASC)
- 研究機構
根據最終用戶,聽力設備市場分為醫院、門診手術中心(ASC)和研究機構。
聽力學設備市場區域分析/洞察
對聽力學設備市場進行了分析,並按國家、產品、類型、技術、銷售管道、疾病類型和最終用戶提供了市場規模洞察和趨勢,如上所述。
聽力學設備市場報告涵蓋的國家有:北美洲的美國、加拿大和墨西哥,歐洲的德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其,歐洲其他地區,中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓,亞太地區(APAC)的其他地區,沙烏地阿拉伯、阿聯酋、其他國家、歐洲的非洲國家,其他
由於聽力系統的進步、聽力學家數量的增加以及該地區現有供應商引入創新的數位平台,北美在聽力設備市場佔據主導地位。
由於老年人口不斷增加以及與年齡相關的聽力問題、醫療保健基礎設施不斷改善、醫療保健支出不斷增加以及產品知名度不斷提高,預計亞太地區將在 2022 年至 2029 年的預測期內出現顯著增長。
報告的國家部分還提供了各個市場的影響因素以及國內市場監管變化,這些變化會影響市場的當前和未來趨勢。下游和上游價值鏈分析、技術趨勢、波特五力模型分析以及案例研究等數據點是預測各國市場狀況的一些指標。此外,在對國家/地區數據進行預測分析時,還考慮了全球品牌的存在和可用性,以及它們因本土和國內品牌的激烈競爭或稀缺而面臨的挑戰,國內關稅和貿易路線的影響。
醫療保健基礎設施成長安裝基礎和新技術滲透
聽力學設備市場也為您提供詳細的市場分析,涵蓋各國資本設備醫療支出的成長情況、聽力學設備市場中各類產品的安裝基數、生命線曲線技術的影響以及醫療監管環境的變化及其對聽力學設備市場的影響。數據涵蓋2010年至2020年的歷史時期。
競爭格局和聽力學設備市場份額分析
聽力學設備市場競爭格局提供了按競爭對手劃分的詳細資訊。詳細資訊包括公司概況、公司財務狀況、收入、市場潛力、研發投入、新市場計劃、全球佈局、生產基地和設施、生產能力、公司優勢和劣勢、產品發布、產品寬度和廣度以及應用主導地位。以上提供的數據僅與公司在聽力學設備市場的重點相關。
聽力學設備市場的一些主要參與者包括 Demant A/S、GN Store Nord A/S、Sonova、Starkey Laboratories, Inc.、Siemens、Natus Medical Incorporated、MED-EL Medical Electronics、MedRx、Benson Medical、Medtronic、MicroTech、Cochlear Ltd.、WS Audi A/S、Advances、Advancet 和 Nurotechology 和 Nurotechology, Ox1 Co. Ltd. 等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL AUDIOLOGY DEVICES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL AUDIOLOGY DEVICES MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VALUE AND VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL AUDIOLOGY DEVICES MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 EPIMEDIOLOGY
5.1 PREVALENCE OF SENSORINEURAL HEARING LOSS, BY AGE GROUP
5.2 PREVALENCE OF CONDUCTIVE HEARING LOSS, BY AGE GROUP
6 PREMIUM INSIGHTS
6.1 PESTAL ANALYSIS
6.2 PORTER’S FIVE FORCES
6.3 KEY STRATEGIC INITIATIVES
6.3.1 HEARING CONSERVATION PROGRAMS
6.3.2 OTHERS
6.4 TECHNOLOGICAL INOVATIONS
7 INDUSTRY INSIGHTS
8 REGULATORY FRAMWORK
9 GLOBAL AUDIOLOGY DEVICES MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 HEARING AID DEVICES
9.2.1 RECEIVER-IN-THE-EAR HEARING AIDS
9.2.1.1. MICRO RIC/RITE
9.2.1.1.1. RECHARGABLE
9.2.1.1.1.1 MARKET VALUE (USD MN)
9.2.1.1.1.2 MARKET VOLUME (UNITS)
9.2.1.1.1.3 AVERAGE SELLINF PRICE (USD)
9.2.1.1.2. NON-RECHARGABLE
9.2.1.1.2.1 MARKET VALUE (USD MN)
9.2.1.1.2.2 MARKET VOLUME (UNITS)
9.2.1.1.2.3 AVERAGE SELLINF PRICE (USD)
9.2.1.2. STANDARD RIC/RITE
9.2.1.2.1. RECHARGABLE
9.2.1.2.1.1 MARKET VALUE (USD MN)
9.2.1.2.1.2 MARKET VOLUME (UNITS)
9.2.1.2.1.3 AVERAGE SELLINF PRICE (USD)
9.2.1.2.2. NON-RECHARGABLE
9.2.1.2.2.1 MARKET VALUE (USD MN)
9.2.1.2.2.2 MARKET VOLUME (UNITS)
9.2.1.2.2.3 AVERAGE SELLINF PRICE (USD)
9.2.2 BEHIND-THE-EAR HEARING AIDS
9.2.2.1. MINI BEHIND-THE EAR (BTE)
9.2.2.1.1. RECHARGABLE
9.2.2.1.1.1 MARKET VALUE (USD MN)
9.2.2.1.1.2 MARKET VOLUME (UNITS)
9.2.2.1.1.3 AVERAGE SELLINF PRICE (USD)
9.2.2.1.2. NON-RECHARGABLE
9.2.2.1.2.1 MARKET VALUE (USD MN)
9.2.2.1.2.2 MARKET VOLUME (UNITS)
9.2.2.1.2.3 AVERAGE SELLINF PRICE (USD)
9.2.2.2. STANDARD BEHIND-THE EAR (BTE)
9.2.2.2.1. RECHARGABLE
9.2.2.2.1.1 MARKET VALUE (USD MN)
9.2.2.2.1.2 MARKET VOLUME (UNITS)
9.2.2.2.1.3 AVERAGE SELLINF PRICE (USD)
9.2.2.2.2. NON-RECHARGABLE
9.2.2.2.2.1 MARKET VALUE (USD MN)
9.2.2.2.2.2 MARKET VOLUME (UNITS)
9.2.2.2.2.3 AVERAGE SELLINF PRICE (USD)
9.2.2.3. POWER BEHIND-THE EAR (BTE)
9.2.2.3.1. RECHARGABLE
9.2.2.3.1.1 MARKET VALUE (USD MN)
9.2.2.3.1.2 MARKET VOLUME (UNITS)
9.2.2.3.1.3 AVERAGE SELLINF PRICE (USD)
9.2.2.3.2. NON-RECHARGABLE
9.2.2.3.2.1 MARKET VALUE (USD MN)
9.2.2.3.2.2 MARKET VOLUME (UNITS)
9.2.2.3.2.3 AVERAGE SELLINF PRICE (USD)
9.2.2.4. SINGLE-SIDED HEARING
9.2.2.4.1. RECHARGABLE
9.2.2.4.1.1 MARKET VALUE (USD MN)
9.2.2.4.1.2 MARKET VOLUME (UNITS)
9.2.2.4.1.3 AVERAGE SELLINF PRICE (USD)
9.2.2.4.2. NON-RECHARGABLE
9.2.2.4.2.1 MARKET VALUE (USD MN)
9.2.2.4.2.2 MARKET VOLUME (UNITS)
9.2.2.4.2.3 AVERAGE SELLINF PRICE (USD)
9.2.3 IN-THE-EAR HEARING AIDS
9.2.3.1. INVISIBLE-IN-THE CANAL (IIC)
9.2.3.1.1. RECHARGABLE
9.2.3.1.1.1 MARKET VALUE (USD MN)
9.2.3.1.1.2 MARKET VOLUME (UNITS)
9.2.3.1.1.3 AVERAGE SELLINF PRICE (USD)
9.2.3.1.2. NON-RECHARGABLE
9.2.3.1.2.1 MARKET VALUE (USD MN)
9.2.3.1.2.2 MARKET VOLUME (UNITS)
9.2.3.1.2.3 AVERAGE SELLINF PRICE (USD)
9.2.3.2. COMPLETELY-IN-THE CANAL (CIC)
9.2.3.2.1. RECHARGABLE
9.2.3.2.1.1 MARKET VALUE (USD MN)
9.2.3.2.1.2 MARKET VOLUME (UNITS)
9.2.3.2.1.3 AVERAGE SELLINF PRICE (USD)
9.2.3.2.2. NON-RECHARGABLE
9.2.3.2.2.1 MARKET VALUE (USD MN)
9.2.3.2.2.2 MARKET VOLUME (UNITS)
9.2.3.2.2.3 AVERAGE SELLINF PRICE (USD)
9.2.3.3. IN-THE CANAL (ITC)
9.2.3.3.1. RECHARGABLE
9.2.3.3.1.1 MARKET VALUE (USD MN)
9.2.3.3.1.2 MARKET VOLUME (UNITS)
9.2.3.3.1.3 AVERAGE SELLINF PRICE (USD)
9.2.3.3.2. NON-RECHARGABLE
9.2.3.3.2.1 MARKET VALUE (USD MN)
9.2.3.3.2.2 MARKET VOLUME (UNITS)
9.2.3.3.2.3 AVERAGE SELLINF PRICE (USD)
9.2.3.4. MINI-CANAL (MC) HEARING AIDS
9.2.3.4.1. RECHARGABLE
9.2.3.4.1.1 MARKET VALUE (USD MN)
9.2.3.4.1.2 MARKET VOLUME (UNITS)
9.2.3.4.1.3 AVERAGE SELLINF PRICE (USD)
9.2.3.4.2. NON-RECHARGABLE
9.2.3.4.2.1 MARKET VALUE (USD MN)
9.2.3.4.2.2 MARKET VOLUME (UNITS)
9.2.3.4.2.3 AVERAGE SELLINF PRICE (USD)
9.2.3.5. MICROPHONE-IN-HELIX (MIH) HEARING AIDS
9.2.3.5.1. RECHARGABLE
9.2.3.5.1.1 MARKET VALUE (USD MN)
9.2.3.5.1.2 MARKET VOLUME (UNITS)
9.2.3.5.1.3 AVERAGE SELLINF PRICE (USD)
9.2.3.5.2. NON-RECHARGABLE
9.2.3.5.2.1 MARKET VALUE (USD MN)
9.2.3.5.2.2 MARKET VOLUME (UNITS)
9.2.3.5.2.3 AVERAGE SELLINF PRICE (USD)
9.2.4 OTHER HEARING AID DEVICES
9.3 HEARING IMPLANTS
9.3.1 COCHLEAR IMPLANTS
9.3.1.1. BY PROCESSOR
9.3.1.1.1. BEHIND THE EAR PROCESSOR
9.3.1.1.1.1 MARKET VALUE (USD MN)
9.3.1.1.1.2 MARKET VOLUME (UNITS)
9.3.1.1.1.3 AVERAGE SELLINF PRICE (USD)
9.3.1.1.2. BODY WORN PROCESSOR
9.3.1.1.2.1 MARKET VALUE (USD MN)
9.3.1.1.2.2 MARKET VOLUME (UNITS)
9.3.1.1.2.3 AVERAGE SELLINF PRICE (USD)
9.3.1.2. BY TYPE
9.3.1.2.1. ABUTEMENT
9.3.1.2.2. MAGNET
9.3.1.2.3. HEADBAND
9.3.1.3. BY PPS RATE
9.3.1.3.1. 32000 PPS
9.3.1.3.2. 51000 PPS
9.3.1.3.3. 82000 PPS
9.3.1.4. BY BATTERY
9.3.1.4.1. RECHARGEABLE
9.3.1.4.2. NON RECHARGEABLE
9.3.2 BONE CONDUCTION DEVICES
9.3.2.1. BY PROCESSOR
9.3.2.1.1. BEHIND THE EAR PROCESSOR
9.3.2.1.1.1 MARKET VALUE (USD MN)
9.3.2.1.1.2 MARKET VOLUME (UNITS)
9.3.2.1.1.3 AVERAGE SELLINF PRICE (USD)
9.3.2.1.2. BODY WORN PROCESSOR
9.3.2.1.2.1 MARKET VALUE (USD MN)
9.3.2.1.2.2 MARKET VOLUME (UNITS)
9.3.2.1.2.3 AVERAGE SELLINF PRICE (USD)
9.3.2.2. BY TYPE
9.3.2.2.1. ABUTEMENT (PERCUTANEOUS)
9.3.2.2.2. HEADBANDS
9.3.2.2.3. MAGNETS (TRANSCUTANEOUS)
9.3.2.3. BY BATTERY
9.3.2.3.1. RECHARGEABLE
9.3.2.3.2. NON RECHARGEABLE
9.3.3 MIDDLE EAR IMPLANTS
9.3.3.1. BY PROCESSOR
9.3.3.1.1. BEHIND THE EAR PROCESSOR
9.3.3.1.1.1 MARKET VALUE (USD MN)
9.3.3.1.1.2 MARKET VOLUME (UNITS)
9.3.3.1.1.3 AVERAGE SELLINF PRICE (USD)
9.3.3.1.2. BODY WORN PROCESSOR
9.3.3.1.2.1 MARKET VALUE (USD MN)
9.3.3.1.2.2 MARKET VOLUME (UNITS)
9.3.3.1.2.3 AVERAGE SELLINF PRICE (USD)
9.3.3.2. BY TRANSDUCERS
9.3.3.2.1. PIEZOELECTRIC
9.3.3.2.2. ELECTROMAGNETIC
9.3.4 AUDITORY BRAIN STEM IMPLANTS
9.3.4.1. BYB PROCESSOR
9.3.4.1.1. BEHIND THE EAR PROCESSOR
9.3.4.1.1.1 MARKET VALUE (USD MN)
9.3.4.1.1.2 MARKET VOLUME (UNITS)
9.3.4.1.1.3 AVERAGE SELLINF PRICE (USD)
9.3.4.1.2. BODY WORN PROCESSOR
9.3.4.1.2.1 MARKET VALUE (USD MN)
9.3.4.1.2.2 MARKET VOLUME (UNITS)
9.3.4.1.2.3 AVERAGE SELLINF PRICE (USD)
9.3.4.2. BY ABI CHANNEL
9.3.4.2.1. SINGLE CHANNEL ABI SYSTEM
9.3.4.2.2. MULTI CHANNEL ABI SYSTEM
9.4 HEARING AID ACCESSORIES
9.4.1 HEARING AID BATTERIES
9.4.1.1. MARKET VALUE (USD MN)
9.4.1.2. MARKET VOLUME (UNITS)
9.4.1.3. AVERAGE SELLINF PRICE (USD)
9.4.2 WIRELESS ACCESSORIES
9.4.2.1. MICROPHONE
9.4.2.1.1. MARKET VALUE (USD MN)
9.4.2.1.2. MARKET VOLUME (UNITS)
9.4.2.1.3. AVERAGE SELLINF PRICE (USD)
9.4.2.2. RECEIVER
9.4.2.2.1. MARKET VALUE (USD MN)
9.4.2.2.2. MARKET VOLUME (UNITS)
9.4.2.2.3. AVERAGE SELLINF PRICE (USD)
9.4.2.3. REMOTE
9.4.2.3.1. MINI REMOTE MICROPHONE
9.4.2.3.1.1 MARKET VALUE (USD MN)
9.4.2.3.1.2 MARKET VOLUME (UNITS)
9.4.2.3.1.3 AVERAGE SELLINF PRICE (USD)
9.4.2.3.2. STANDARD REMOTE MICROPHONE
9.4.2.3.2.1 MARKET VALUE (USD MN)
9.4.2.3.2.2 MARKET VOLUME (UNITS)
9.4.2.3.2.3 AVERAGE SELLINF PRICE (USD)
9.4.3 HEARING PROTECTION
9.4.3.1. MARKET VALUE (USD MN)
9.4.3.2. MARKET VOLUME (UNITS)
9.4.3.3. AVERAGE SELLINF PRICE (USD)
9.4.4 WAX GUARD FILTERS
9.4.4.1. MARKET VALUE (USD MN)
9.4.4.2. MARKET VOLUME (UNITS)
9.4.4.3. AVERAGE SELLINF PRICE (USD)
9.4.5 OTHERS
10 GLOBAL AUDIOLOGY DEVICES MARKET, BY HEARING LOSS TYPE
10.1 OVERVIEW
10.2 SENSORINEURAL HEARING LOSS
10.2.1 HEARING AID DEVICES
10.2.1.1. RECEIVER-IN-THE-EAR HEARING AIDS
10.2.1.2. BEHIND-THE-EAR HEARING AIDS
10.2.1.3. IN-THE-EAR HEARING AIDS
10.2.1.4. OTHER HEARING AID DEVICES
10.2.2 HEARING IMPLANTS
10.2.2.1. COCHLEAR IMPLANTS
10.2.2.2. BONE CONDUCTION DEVICES
10.2.2.3. MIDDLE EAR IMPLANTS
10.2.2.4. AUDITORY BRAIN STEM IMPLANTS
10.2.3 HEARING AID ACCESSORIES
10.2.3.1. HEARING AID BATTERIES
10.2.3.2. WIRELESS ACCESSORIES
10.2.3.3. HEARING PROTECTION
10.2.3.4. WAX GUARD FILTERS
10.2.3.5. OTHERS
10.3 CONDUCTIVE HEARING LOSS
10.3.1 HEARING AID DEVICES
10.3.1.1. RECEIVER-IN-THE-EAR HEARING AIDS
10.3.1.2. BEHIND-THE-EAR HEARING AIDS
10.3.1.3. IN-THE-EAR HEARING AIDS
10.3.1.4. OTHER HEARING AID DEVICES
10.3.2 HEARING IMPLANTS
10.3.2.1. COCHLEAR IMPLANTS
10.3.2.2. BONE CONDUCTION DEVICES
10.3.2.3. MIDDLE EAR IMPLANTS
10.3.2.4. AUDITORY BRAIN STEM IMPLANTS
10.3.3 HEARING AID ACCESSORIES
10.3.3.1. HEARING AID BATTERIES
10.3.3.2. WIRELESS ACCESSORIES
10.3.3.3. HEARING PROTECTION
10.3.3.4. WAX GUARD FILTERS
10.3.3.5. OTHERS
11 GLOBAL AUDIOLOGY DEVICES MARKET, BY PATIENT TYPE
11.1 OVERVIEW
11.2 GERIATRIC
11.3 ADULTS
11.4 PEDIATRICS
12 GLOBAL AUDIOLOGY DEVICES MARKET, BY TECHNOLOGY
12.1 OVERVIEW
12.2 DIGITAL SIGNAL PROCESSING
12.3 SMARTPHONE COMPATIBLE
12.4 ARTIFICIAL INTELLIGENCE
12.5 COMPRESSION
12.6 FM COMPATIBILITY
12.7 FEEDBACK MANAGEMENT SYSTEM
12.8 NOISE REDUCTION
12.8.1 DIGITAL NOISE REDUCTION
12.8.2 IMPULSE NOISE REDUCTION
12.8.3 WIND NOISE REDUCTION
12.9 DIRECTIONAL MICROPHONE SYSTEMS
12.1 DATA LOGGING
12.11 TELECOIL
12.12 OTHERS
13 GLOBAL AUDIOLOGY DEVICES MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 CLINICS
13.3.1 AUDIOLOGY CLINICS
13.3.2 ENT CLINICS
13.4 HOME CARE SETTINGS
13.5 OTHERS
14 GLOBAL AUDIOLOGY DEVICES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL PHARMACY
14.4 HOSPITAL PHARMACY
14.5 ONLINE SALES
14.6 OTHERS
15 GLOBAL AUDIOLOGY DEVICES MARKET, BY GEOGRAPHY
GLOBAL AUDIOLOGY DEVICES MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.1.1. U.S. AUDIOLOGY DEVICES MARKET, BY PRODUCT TYPE
15.1.1.2. U.S. AUDIOLOGY DEVICES MARKET, BY HEARING LOSS TYPE
15.1.1.3. U.S. AUDIOLOGY DEVICES MARKET, BY PATIENT TYPE
15.1.1.4. U.S. AUDIOLOGY DEVICES MARKET, BY TECHNOLOGY
15.1.1.5. U.S. AUDIOLOGY DEVICES MARKET, BY END USER
15.1.1.6. U.S. AUDIOLOGY DEVICES MARKET, BY DISTRIBUTION CHANNEL
15.1.2 CANADA
15.1.3 MEXICO
15.2 EUROPE
15.2.1 GERMANY
15.2.2 FRANCE
15.2.3 U.K.
15.2.4 ITALY
15.2.5 SPAIN
15.2.6 RUSSIA
15.2.7 TURKEY
15.2.8 BELGIUM
15.2.9 NETHERLANDS
15.2.10 SWITZERLAND
15.2.11 REST OF EUROPE
15.3 ASIA-PACIFIC
15.3.1 JAPAN
15.3.2 CHINA
15.3.3 SOUTH KOREA
15.3.4 INDIA
15.3.5 AUSTRALIA
15.3.6 SINGAPORE
15.3.7 THAILAND
15.3.8 MALAYSIA
15.3.9 INDONESIA
15.3.10 PHILIPPINES
15.3.11 REST OF ASIA-PACIFIC
15.4 SOUTH AMERICA
15.4.1 BRAZIL
15.4.2 ARGENTINA
15.4.3 REST OF SOUTH AMERICA
15.5 MIDDLE EAST AND AFRICA
15.5.1 SOUTH AFRICA
15.5.2 SAUDI ARABIA
15.5.3 UAE
15.5.4 EGYPT
15.5.5 ISRAEL
15.5.6 REST OF MIDDLE EAST AND AFRICA
15.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
16 GLOBAL AUDIOLOGY DEVICES MARKET, SWOT AND DBMR ANALYSIS
17 GLOBAL AUDIOLOGY DEVICES MARKET, COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: GLOBAL
17.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
17.3 COMPANY SHARE ANALYSIS: EUROPE
17.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17.5 MERGERS & ACQUISITIONS
17.6 NEW PRODUCT DEVELOPMENT & APPROVALS
17.7 EXPANSIONS
17.8 REGULATORY CHANGES
17.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
18 GLOBAL AUDIOLOGY DEVICES MARKET, COMPANY PROFILE
18.1 SONOVA GROUP
18.1.1 COMPANY OVERVIEW
18.1.2 REVENUE ANALYSIS
18.1.3 GEOGRAPHIC PRESENCE
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 DEMANT A/S
18.2.1 COMPANY OVERVIEW
18.2.2 REVENUE ANALYSIS
18.2.3 GEOGRAPHIC PRESENCE
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GN STORE NORD A/S
18.3.1 COMPANY OVERVIEW
18.3.2 REVENUE ANALYSIS
18.3.3 GEOGRAPHIC PRESENCE
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENTS
18.4 COCHLEAR LTD.
18.4.1 COMPANY OVERVIEW
18.4.2 REVENUE ANALYSIS
18.4.3 GEOGRAPHIC PRESENCE
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 STARKEY LABORATORIES, INC.
18.5.1 COMPANY OVERVIEW
18.5.2 REVENUE ANALYSIS
18.5.3 GEOGRAPHIC PRESENCE
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
18.6 AUDINA HEARING INSTRUMENTS, INC.
18.6.1 COMPANY OVERVIEW
18.6.2 REVENUE ANALYSIS
18.6.3 GEOGRAPHIC PRESENCE
18.6.4 PRODUCT PORTFOLIO
18.6.5 RECENT DEVELOPMENTS
18.7 OTICON
18.7.1 COMPANY OVERVIEW
18.7.2 REVENUE ANALYSIS
18.7.3 GEOGRAPHIC PRESENCE
18.7.4 PRODUCT PORTFOLIO
18.7.5 RECENT DEVELOPMENTS
18.8 WS AUDIOLOGY A/S
18.8.1 COMPANY OVERVIEW
18.8.2 REVENUE ANALYSIS
18.8.3 GEOGRAPHIC PRESENCE
18.8.4 PRODUCT PORTFOLIO
18.8.5 RECENT DEVELOPMENTS
18.9 UNITRON
18.9.1 COMPANY OVERVIEW
18.9.2 REVENUE ANALYSIS
18.9.3 GEOGRAPHIC PRESENCE
18.9.4 PRODUCT PORTFOLIO
18.9.5 RECENT DEVELOPMENTS
18.1 EARGO INC.
18.10.1 COMPANY OVERVIEW
18.10.2 REVENUE ANALYSIS
18.10.3 GEOGRAPHIC PRESENCE
18.10.4 PRODUCT PORTFOLIO
18.10.5 RECENT DEVELOPMENTS
18.10.6 MDHEARING
18.10.7 COMPANY OVERVIEW
18.10.8 REVENUE ANALYSIS
18.10.9 GEOGRAPHIC PRESENCE
18.10.10 PRODUCT PORTFOLIO
18.10.11 RECENT DEVELOPMENTS
18.11 MEDTRONIC
18.11.1 COMPANY OVERVIEW
18.11.2 REVENUE ANALYSIS
18.11.3 GEOGRAPHIC PRESENCE
18.11.4 PRODUCT PORTFOLIO
18.11.5 RECENT DEVELOPMENTS
18.12 ADVANCED BIONICS CORPORATION
18.12.1 COMPANY OVERVIEW
18.12.2 REVENUE ANALYSIS
18.12.3 GEOGRAPHIC PRESENCE
18.12.4 PRODUCT PORTFOLIO
18.12.5 RECENT DEVELOPMENTS
18.13 BOSTON SCIENTIFIC
18.13.1 COMPANY OVERVIEW
18.13.2 REVENUE ANALYSIS
18.13.3 GEOGRAPHIC PRESENCE
18.13.4 PRODUCT PORTFOLIO
18.13.5 RECENT DEVELOPMENTS
18.14 BIOTRONIK
18.14.1 COMPANY OVERVIEW
18.14.2 REVENUE ANALYSIS
18.14.3 GEOGRAPHIC PRESENCE
18.14.4 PRODUCT PORTFOLIO
18.14.5 RECENT DEVELOPMENTS
18.15 LIVA NOVA PLC
18.15.1 COMPANY OVERVIEW
18.15.2 REVENUE ANALYSIS
18.15.3 GEOGRAPHIC PRESENCE
18.15.4 PRODUCT PORTFOLIO
18.15.5 RECENT DEVELOPMENTS
18.16 BERNAFON
18.16.1 COMPANY OVERVIEW
18.16.2 REVENUE ANALYSIS
18.16.3 GEOGRAPHIC PRESENCE
18.16.4 PRODUCT PORTFOLIO
18.16.5 RECENT DEVELOPEMENTS
18.17 NATUS MEDICAL INCORPORATED
18.17.1 COMPANY OVERVIEW
18.17.2 REVENUE ANALYSIS
18.17.3 GEOGRAPHIC PRESENCE
18.17.4 PRODUCT PORTFOLIO
18.17.5 RECENT DEVELOPEMENTS
18.18 SIEMENS AG
18.18.1 COMPANY OVERVIEW
18.18.2 REVENUE ANALYSIS
18.18.3 GEOGRAPHIC PRESENCE
18.18.4 PRODUCT PORTFOLIO
18.18.5 RECENT DEVELOPEMENTS
18.19 SONIC INNOVATIONS, INC.,
18.19.1 COMPANY OVERVIEW
18.19.2 REVENUE ANALYSIS
18.19.3 GEOGRAPHIC PRESENCE
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPEMENTS
18.19.6 RECENT DEVELOPMENTS
18.2 SIVANTOS PTE. LTD.
18.20.1 COMPANY OVERVIEW
18.20.2 REVENUE ANALYSIS
18.20.3 GEOGRAPHIC PRESENCE
18.20.4 PRODUCT PORTFOLIO
18.20.5 RECENT DEVELOPMENTS
18.21 AMPLIFON
18.21.1 COMPANY OVERVIEW
18.21.2 REVENUE ANALYSIS
18.21.3 GEOGRAPHIC PRESENCE
18.21.4 PRODUCT PORTFOLIO
18.21.5 RECENT DEVELOPMENTS
18.22 AUSTAR
18.22.1 COMPANY OVERVIEW
18.22.2 REVENUE ANALYSIS
18.22.3 GEOGRAPHIC PRESENCE
18.22.4 PRODUCT PORTFOLIO
18.22.5 RECENT DEVELOPMENTS
18.23 HORENTEK HEARING DIAGNOSTICS
18.23.1 COMPANY OVERVIEW
18.23.2 REVENUE ANALYSIS
18.23.3 GEOGRAPHIC PRESENCE
18.23.4 PRODUCT PORTFOLIO
18.23.5 RECENT DEVELOPMENTS
18.24 ZOUNDS HEARING
18.24.1 COMPANY OVERVIEW
18.24.2 REVENUE ANALYSIS
18.24.3 GEOGRAPHIC PRESENCE
18.24.4 PRODUCT PORTFOLIO
18.24.5 RECENT DEVELOPMENTS
18.25 SEBOTEK HEARING SYSTEMS, LLC.
18.25.1 COMPANY OVERVIEW
18.25.2 REVENUE ANALYSIS
18.25.3 GEOGRAPHIC PRESENCE
18.25.4 PRODUCT PORTFOLIO
18.25.5 RECENT DEVELOPMENTS
18.26 NANO HEARING AIDS
18.26.1 COMPANY OVERVIEW
18.26.2 REVENUE ANALYSIS
18.26.3 GEOGRAPHIC PRESENCE
18.26.4 PRODUCT PORTFOLIO
18.26.5 RECENT DEVELOPMENTS
18.27 EARLENS CORP.
18.27.1 COMPANY OVERVIEW
18.27.2 REVENUE ANALYSIS
18.27.3 GEOGRAPHIC PRESENCE
18.27.4 PRODUCT PORTFOLIO
18.27.5 RECENT DEVELOPMENTS
18.28 LIVELY HEARING CORPORATION
18.28.1 COMPANY OVERVIEW
18.28.2 REVENUE ANALYSIS
18.28.3 GEOGRAPHIC PRESENCE
18.28.4 PRODUCT PORTFOLIO
18.28.5 RECENT DEVELOPMENTS
18.29 BELTONE GROUP
18.29.1 COMPANY OVERVIEW
18.29.2 REVENUE ANALYSIS
18.29.3 GEOGRAPHIC PRESENCE
18.29.4 PRODUCT PORTFOLIO
18.29.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST Conclusion
19 RELATED REPORTS
20 CONCLUSION
21 QUESTIONNAIRE
22 ABOUT DATA BRIDGE MARKET RESEARCH

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。