Global Hernia Mesh Repair Devices Market
市场规模(十亿美元)
CAGR :
% 
 USD
3.90 Billion
USD
5.16 Billion
2024
2032
USD
3.90 Billion
USD
5.16 Billion
2024
2032
| 2025 –2032 | |
| USD 3.90 Billion | |
| USD 5.16 Billion | |
|
|
|
|
Global Hernia Mesh Repair Devices Market Segmentation, By Product (Mesh and Mesh Fixators), Hernia Type (Inguinal Hernia, Incisional/ Ventral Hernia, Umbilical Hernia, Femoral Hernia, Hiatal Hernia, Parastomal Hernia, and Others), End Users (Ambulatory Surgical Centres, Clinics, Hospitals, and Others) - Industry Trends and Forecast to 2032
Hernia Mesh Repair Devices Market Size
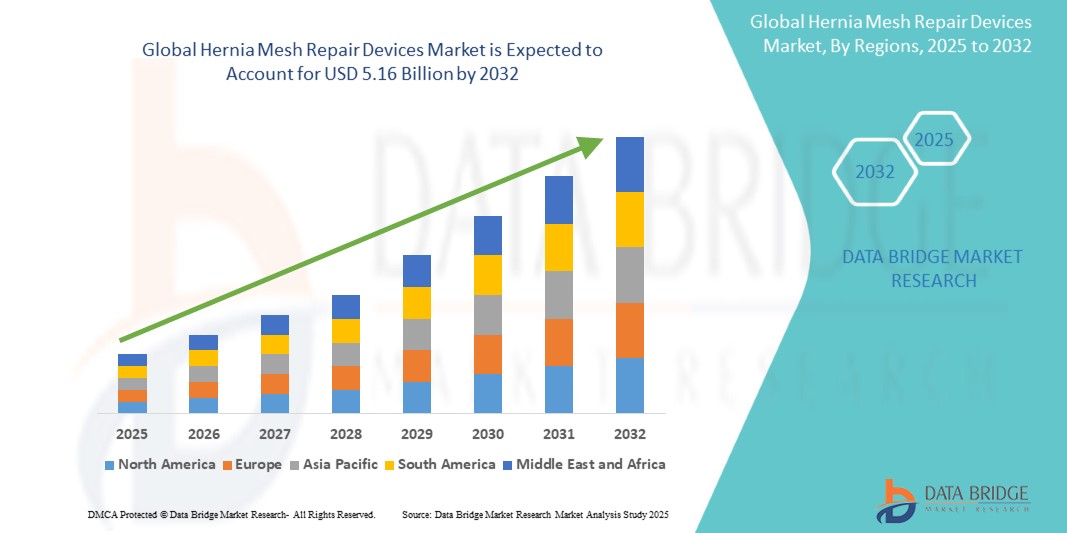
- The global hernia mesh repair devices market size was valued atUSD 3.90 billion in 2024and is expected to reachUSD 5.16 billion by 2032, at aCAGR of 3.55%during the forecast period
- This growth is driven by factors such as the aging population, increasing prevalence of eye diseases, and advancements in ophthalmic technology
Hernia Mesh Repair Devices Market Analysis
- Hernia mesh repair devices refer to medical implants used in the surgical treatment of hernias
- These devices are typically made from synthetic materials such as polypropylene and are designed to reinforce weakened tissue or close gaps in the abdominal wall where hernias occur
- The mesh helps to reduce the recurrence of hernias by providing support and facilitating tissue growth around the implant
- North America is expected to dominate the hernia mesh repair devices market with 50.9 due to well-established healthcare infrastructure, high surgical volumes, and a strong focus on innovation in medical devices
- Asia-pacific is expected to be the fastest growing region in the hernia mesh repair devices market during the forecast period due to rising incidence of hernias, increasing healthcare expenditures, and advancements in surgical techniques
- Synthetic mesh segment is expected to dominate the market with a market share of 65.1% due to its widespread adoption of synthetic materials due to their desirable properties and proven effectiveness
Report Scope andHernia Mesh Repair Devices Market Segmentation
|
Attributes |
Hernia Mesh Repair Devices KeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Hernia Mesh Repair Devices Market Trends
“Surge in Mesh Utilization for Hernia Repairs”
- Hernia repair surgeries incorporate mesh, underscoring its critical role in modern surgical practices
- Mesh implants provide structural support, reducing recurrence rates and improving long-term patient outcomes
- Innovations such as self-fixating meshes and bioresorbable materials are gaining traction, offering benefits such as reduced postoperative pain and quicker recovery times
- The rise in laparoscopic surgeries has increased the demand for mesh devices, as they facilitate smaller incisions and faster healing
- Recent FDA clearances for new mesh products, such as TELA Bio's OviTex PRS Long-Term Resorbable mesh, reflect growing confidence and innovation in the field
Hernia Mesh Repair Devices Market Dynamics
Driver
“Rising Prevalence of Hernias”
- An increase in the elderly demographic is contributing to a higher incidence of hernias
- Obesity and sedentary lifestyles are significant risk factors, leading to more individuals requiring hernia repair
- For instance, China reports approximately 1.2 million inguinal hernia surgeries annually, highlighting the widespread nature of the condition Improved access to healthcare services is enabling more people to seek treatment for hernias
- Developing economies are witnessing increased healthcare investments, facilitating better treatment options for hernia patients
Opportunity
“Expansion into Emerging Markets”
- Regions such as Asia-Pacific and Latin America present significant growth opportunities due to rising healthcare awareness and infrastructure development
- Affordable mesh products can cater to the budget constraints of these regions, expanding market reach
- Establishing production facilities in emerging markets can reduce costs and improve supply chain efficiency
- Supportive policies and investments in healthcare are creating a conducive environment for market growth
- Increasing awareness and acceptance of surgical interventions are driving demand for hernia repair devices
Restraint/Challenge
“Complications and Safety Concerns”
- Complications such as infections, chronic pain, and mesh migration can arise, affecting patient recovery
- Stringent regulations and safety concerns have led to recalls and lawsuits, impacting market dynamics
- Certain mesh materials may not be suitable for all patients, necessitating personalized treatment approaches
- The long-term impact of mesh implants is still under study, raising concerns among healthcare providers and patients
- High-quality meshes can be expensive, limiting accessibility for some patient populations
Hernia Mesh Repair Devices Market Scope
The market is segmented on the basis of product, hernia type, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product |
|
|
By Hernia Type |
|
|
By End Users |
|
In 2025, the synthetic mesh is projected to dominate the market with a largest share in product segment
The synthetic mesh segment is expected to dominate the Hernia Mesh Repair Devices market with the largest share of 65.1% in 2025 due to its widespread adoption of synthetic materials due to their desirable properties and proven effectiveness.
The hospitals is expected to account for the largest share during the forecast period in end user market
In 2025, the hospitals segment is expected to dominate the market with the largest market share of 54.2% due to its high prevalence of surgical procedures performed in these settings and the increasing number of specialized surgical centers focused on hernia repairs.
Hernia Mesh Repair Devices Market Regional Analysis
“North America Holds the Largest Share in the Hernia Mesh Repair Devices Market”
- North America holds a significant share of the global hernia mesh repair devices market, with the U.S. accounting for approximately 50.9% of the market
- Inguinal hernia repair remains the largest revenue-generating segment, while incisional hernia repair is anticipated to register the fastest growth during the forecast period
- Factors such as advanced healthcare infrastructure, a high prevalence of hernia cases, and a growing geriatric population contribute to the dominance of North America in this market.
- Major companies operating in the region include Medtronic, Johnson & Johnson, BD, W.L. Gore & Associates, and COOK Medical
“Asia-Pacific is Projected to Register the HighestCAGR in the Hernia Mesh Repair Devices Market”
- The Asia-Pacific region is experiencing rapid growth in the hernia mesh repair devices market
- Inguinal hernia repair currently leads in revenue generation, while incisional hernia repair is anticipated to exhibit the fastest growth during the forecast period
- South Korea is expected to register the highest CAGR within the region
- Increasing healthcare investments, rising awareness about hernia management, and improving healthcare infrastructure are key factors driving market growth in Asia-Pacific
Hernia Mesh Repair Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Medtronic(U.S.)
- BD(U.S.)
- Cook Group, Inc. (U.S.)
- W. L. Gore & Associates, Inc.(U.S.)
- ETHICON(U.S.)
- Baxter (U.S.)
- Cooper Surgical Inc. (U.S.)
- B. Braun Melsungen AG (Germany)
- Integra LifeSciences (U.S.)
Latest Developments in Global Hernia Mesh Repair Devices Market
- In January 2024,RTI Surgicalcompleted its acquisition of Cook Biotech, a move to strengthen its position in the surgical and regenerative medicine markets. This acquisition allows RTI to expand its portfolio of biologic and synthetic products, enhancing its ability to provide innovative solutions for surgical procedures. Integrating Cook Biotech's expertise in hernia repair and wound management is expected to drive growth and improve patient outcomes
- In April 2024, the company planned to launch a new smaller version of theT-Line Mesh, named the T-Line Mini, which was designed for umbilical hernias and small defects. Biosynthetic and coated composite versions of the T-Line Hernia Mesh were also under development
- In March 2024,TELA Bio, Inc.,a commercial-stage medical technology company specializing in advanced soft-tissue reconstruction solutions, announced the U.S. release of LIQUIFIX FIX8™ Laparoscopic and LIQUIFIX Precision™ Open Hernia Mesh Fixation Devices. LIQUIFIX FIX8 is designed for minimally invasive repair of femoral and inguinal hernias, while LIQUIFIX Precision is intended for open repair of femoral and inguinal hernias
- In January 2022,Cook Biotech Inc. announced a partnership with the European Hernia Society (EHS) to advance hernia repair technologies. This collaboration aims to promote research, education, and clinical practice related to hernia management. By joining forces, Cook Biotech and EHS intend to enhance the understanding of hernia repair procedures and improve patient outcomes through innovative solutions. The partnership underscores a commitment to developing effective treatment options and fostering collaboration within the medical community.
- December 2022 –W. L. Gore & Associates, Inc. partnered with AGC Biologics for protein A-based purification technology, intending to reduce the manufacturing footprint and the clinical manufacturing cost of its products.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。