全球腸躁症 (IBS) 市場,依藥物類型(魯比前列酮、利那洛肽、艾魯沙多林、利福昔明、阿洛司瓊及其他)、疾病類型(便秘型 IBS、腹瀉型IBS、混合型)、分銷管道(醫院藥房、線上藥房、零售藥房)、終端用戶(醫院、診所、家庭護理機構)、國家/地區(美國、加拿大、墨西哥、秘魯、巴西、阿根廷、南美其他地區、德國、義大利、英國、法國、西班牙、荷蘭、比利時、瑞士、土耳其、俄羅斯、匈牙利、立陶宛、奧地利、愛爾蘭、挪威、波蘭、歐洲其他地區、日本、中國、印度、韓國、澳洲、新加坡、馬來西亞、泰國、印尼、菲律賓、越南、亞太其他地區、南非、沙烏地阿拉伯、阿聯酋、科威特、以色列、埃及、中東和非洲其他地區)劃分,產業趨勢及至2029 年的預測
全球腸躁症(IBS)市場分析與洞察
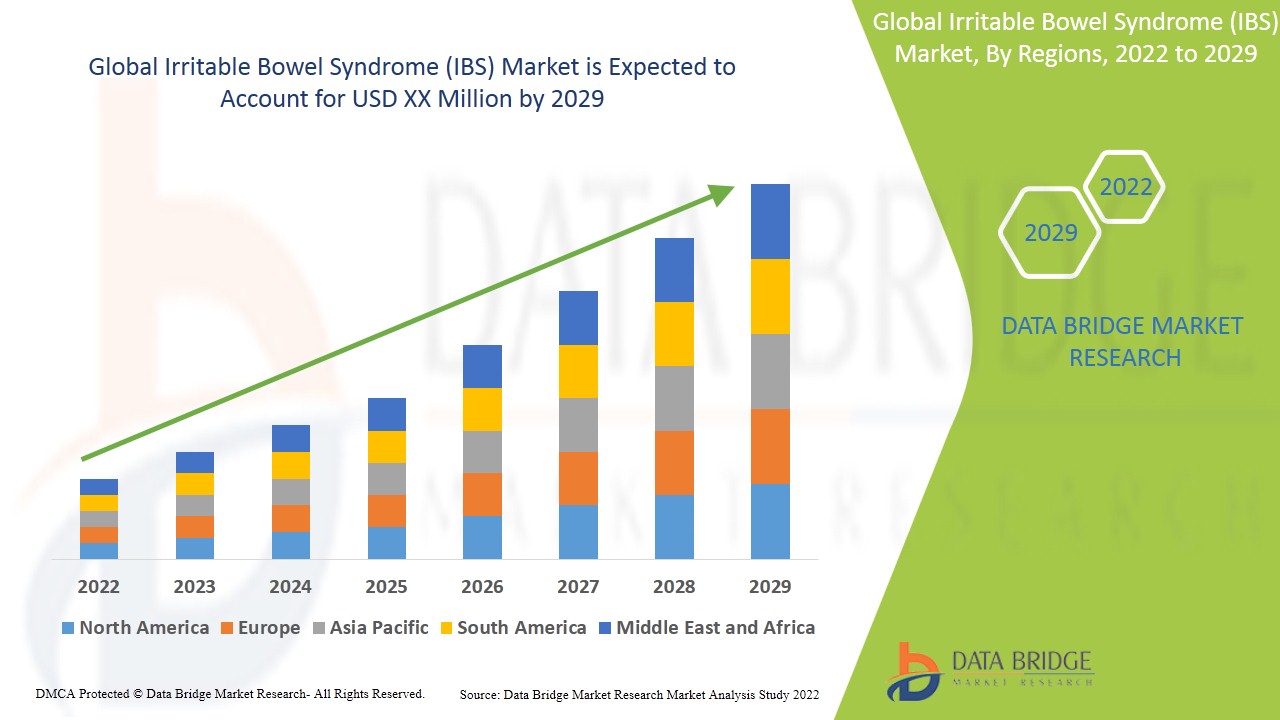
預計2022年至2029年預測期內,腸躁症(IBS)市場將以12.90%的複合年增長率成長。 Data Bridge Market Research發布的腸躁症(IBS)市場報告分析並深入探討了預測期內各種影響因素及其對市場成長的影響。全球慢性病盛行率的上升正在推動腸躁症(IBS)市場的成長。
腸躁症(IBS)是一種常見的胃腸道疾病,主要影響大腸。腹痛、脹氣、便秘或腹瀉、腹脹和痙攣都是IBS的徵兆和症狀。腸躁症分為三種:便秘型腸躁症(IBS-C)、腹瀉型腸躁症(IBS-D)及混合型腸躁症。
全球腸躁症 (IBS) 盛行率的上升是推動腸躁症 (IBS) 市場成長的主要因素之一。快節奏的生活方式導致壓力水平上升,以及人們對腸躁症治療和管理的認識不斷提高,都加速了市場成長。胃腸道疾病和紊亂(如排便習慣改變和腹痛)盛行率的上升,以及不健康飲食習慣的盛行,也進一步影響市場。此外,人口成長、醫療保健支出激增、技術進步、研發投入增加以及老年人口成長也對腸躁症 (IBS) 市場產生正面影響。此外,消費者對能夠增強治療效果的 IBS 產品的需求,也為市場參與者在 2022 年至 2029 年的預測期內提供了獲利機會。
另一方面,缺乏針對腸躁症(IBS)所有症狀的特效療法預計將阻礙市場成長。主要參與者之間的激烈競爭預計將在2022-2029年的預測期內對腸躁症(IBS)市場構成挑戰。
這份腸躁症 (IBS) 市場報告詳細介紹了近期新發展、貿易法規、進出口分析、生產分析、價值鏈優化、市場份額、國內外市場參與者的影響、新興收入來源的機遇分析、市場法規變化、戰略市場成長分析、市場規模、各類別市場成長、應用領域及主導地位、產品審批、產品發布、戰略市場成長分析、市場規模、各類別市場成長、應用領域及主導地位、產品審批、產品發布、策略市場成長分析、市場規模、各類別市場成長、應用領域及主導地位、產品審批、產品發布、策略市場成長分析、地域發展如需了解更多關於腸躁症 (IBS) 市場的信息,請聯繫 Data Bridge Market Research 以獲取分析師簡報,我們的團隊將協助您做出明智的市場決策,以實現市場成長。
全球腸躁症(IBS)市場範圍與市場規模
腸躁症 (IBS) 市場根據藥物類型、藥品版本、分銷管道和最終用戶進行細分。各細分市場的成長情況有助於您分析潛在的成長點和市場策略,確定您的核心應用領域以及目標市場的差異。
- 根據藥物類型,腸躁症 (IBS) 市場可分為魯比前列酮、利那洛肽、艾魯沙多林、利福昔明、阿洛司瓊和其他藥物。
- 根據醫學分類,腸躁症 (IBS) 市場分為便秘型 IBS、腹瀉型 IBS 和混合型 IBS。
- 根據分銷管道,腸躁症 (IBS) 市場分為醫院藥房、網路藥房和零售藥房。
- 根據最終用戶,腸躁症 (IBS) 市場分為醫院藥房、診所、零售藥房和線上銷售。
腸躁症(IBS)市場國家層面分析
對腸躁症 (IBS) 市場進行分析,並按國家、藥物類型、醫療版本、分銷管道和最終用戶提供市場規模信息,如上所述。
全球腸躁症 (IBS)市場報告涵蓋的國家包括:北美洲的美國、加拿大和墨西哥;南美洲的秘魯、巴西、阿根廷和南美洲其他國家;歐洲的德國、義大利、英國、法國、西班牙、荷蘭、比利時、瑞士、土耳其、俄羅斯、匈牙利、立陶宛、奧地利、愛爾蘭、挪威、波蘭和歐洲其他國家;亞太地區的日本、中國、印度、韓國、澳洲、新加坡、馬來西亞、泰國、印尼、菲律賓、越南和亞太地區的其他國家;以及中東和非洲地區的南非、沙烏地阿拉伯、阿聯酋、科威特、以色列、埃及和中東及非洲其他國家。
由於北美地區擁有發達的醫療保健基礎設施,因此該地區在腸躁症(IBS)市場佔據主導地位。亞太地區人口眾多,預計在2022年至2029年的預測期內,該地區將實現高速成長。
報告的國別部分還提供了影響各個市場的因素以及國內市場監管變化,這些因素和變化會影響市場的當前和未來趨勢。新銷售額、替換銷售額、國家人口統計、疾病流行病學和進出口關稅等數據點是預測各國市場前景的主要指標。此外,在對各國數據進行預測分析時,也會考慮全球品牌的市場佔有率和可用性,以及它們因本地品牌競爭激烈或稀缺而面臨的挑戰,以及銷售管道的影響。
患者流行病學分析
腸躁症 (IBS) 市場報告還提供詳細的市場分析,涵蓋患者分析、預後和治療方案。報告中提供的數據變數包括盛行率、發病率、死亡率和依從率等。報告分析了流行病學對市場成長的直接或間接影響,旨在建立更穩健的隊列多元統計模型,從而預測市場在增長期內的發展趨勢。
競爭格局及腸躁症(IBS)市佔率分析
腸躁症 (IBS) 市場競爭格局分析按競爭對手提供詳細資訊。其中包括公司概況、財務狀況、收入、市場潛力、研發投入、新市場拓展計劃、全球佈局、生產基地和設施、產能、公司優勢和劣勢、產品發布、產品線寬度和廣度以及應用領域優勢。以上數據僅與各公司專注於腸躁症 (IBS) 市場的業務相關。
在腸躁症 (IBS) 市場報告中,一些主要參與者包括 Ironwood Pharmaceuticals, Inc.、Allergan、Astellas Pharma, Inc.、Takeda Pharmaceutical Company Limited、AstraZeneca、Sebela Pharmaceuticals Inc.、Synergy Pharmaceuticals In.、Bausch Health、Synthetics Inc.、Synergy Pharmaceuticals In.、Bausch Health、Synthetics Inc. Pharmaceuticals、Mallinckrodt.、Abbott Laboratories、LEXICON PHARMACEUTICALS, INC、GlaxoSmithKline plc、Johnson & Johnson Services, Inc、Ono Pharmaceutical Co., Ltd.、Pfizer Inc、Novartis 和 Sebela Pharmaceuticals Inc. 等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S 5 FORCES
4.2 PESTEL ANALYSIS
4.3 POWDER PRICE
4.4 DEMAND AND SUPPLY ANALYSIS
4.5 TREND FOR WORK
5 EPIDEMIOLOGY
5.1 PREVELENCE OF IRRITABLE BOWEL SYNDROME
5.2 INCIDENCE OF IRRITABLE BOWEL SYNDROME, BY GENDER
5.3 TREATMENT RATE
5.4 MORTALITY RATE
5.5 DRUG ADHERENCE AND THERAPY SWITCH MODEL
5.6 PATEINT TREATMENT SUCCESS RATES
5.7 INCERPTS FROM GLOBAL HEALTH DATA EXCHANGE (GHDX)
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH GASTRENTEROLOGIST
6.8 INTERVIEWS WITH DRUG MANUFACTURERS
6.9 OTHER KOL SNAPSHOTS
7 REGULATORY SCENARIO
8 PIPELINE ANALYSIS
8.1 CLINICAL TRIALS AND PHASE ANALYSIS
8.2 DRUG THERAPY PIPELINE
8.3 PHASE III CANDIDATES
8.4 PHASE II CANDIDATES
8.5 PHASE I CANDIDATES
8.6 OTHERS (PRE-CLINICAL AND RESEARCH)
9 MARKET OVERVIEW
9.1 DRIVERS
9.2 RESTRAINS
9.3 OPPURTUNITY
9.4 CHALLENGES
10 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY TYPE
10.1 OVERVIEW
10.2 IBS WITH CONSTIPATION (IBS-C)
10.3 IBS WITH DIARRHEA (IBS-D)
10.4 MIXED IBS (IBS-M)
10.5 UNSUBTYPED IBS (IBS-U)
11 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY DIAGNOSIS & TREATMENT
11.1 OVERVIEW
11.2 DIAGNOSIS
11.2.1 BLOOD TEST
11.2.1.1. ANEMIA
11.2.1.2. INFECTION
11.2.1.3. DIGESTIVE DISEASES
11.2.1.4. OTHERS
11.2.2 IMAGING TEST
11.2.2.1. COLONOSCOPY
11.2.2.2. X-RAY
11.2.2.3. CT SCAN
11.2.2.4. UPPER ENDOSCOPY
11.2.2.5. OTHERS
11.2.3 STOOL TEST
11.2.4 OTHERS
11.3 TREATMENT
11.3.1 MEDICATION
11.3.1.1. SECRETAGOGUES
11.3.1.1.1. LUBIPROSTONE (AMITIZA)
11.3.1.1.1.1 MARKET SHARE (USD)
11.3.1.1.1.2 MARKET VOLUME (UNIT)
11.3.1.1.1.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.2. LINACLOTIDE (LINZESS)
11.3.1.1.2.1 MARKET SHARE (USD)
11.3.1.1.2.2 MARKET VOLUME (UNIT)
11.3.1.1.2.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.3. PLECANATIDE (TRULANCE)
11.3.1.1.3.1 MARKET SHARE (USD)
11.3.1.1.3.2 MARKET VOLUME (UNIT)
11.3.1.1.3.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.4. TENAPENOR (IBSRELA)
11.3.1.1.4.1 MARKET SHARE (USD)
11.3.1.1.4.2 MARKET VOLUME (UNIT)
11.3.1.1.4.3 AVERAGE SELLING PRICE (USD)
11.3.1.2. LAXATIVE
11.3.1.2.1. BY TYPE
11.3.1.2.1.1 OSMOTIC
11.3.1.2.1.1.1. PEG
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.1.2. LACTULOSE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.1.3. MILK OF MAGNESIA
11.3.1.2.1.1.4. OTHERS
11.3.1.2.1.2 FIBER SUPPLEMENTS
11.3.1.2.1.2.1. PSYLLIUM
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.2.2. ACACIA
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.2.3. THORNE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.2.4. OTHERS
11.3.1.2.1.3 STIMULANT
11.3.1.2.1.3.1. SENNA CASCARA
11.3.1.2.1.3.2. BISACODYL
11.3.1.2.1.3.3. OTHERS
11.3.1.2.2. BY DRUG TYPE
11.3.1.2.2.1 GENERIC
11.3.1.2.2.2 BRANDED
11.3.1.2.2.2.1. MIRALAX
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.2. DULCOLAX
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.3. CORRECTOL
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.4. OTHERS
11.3.1.3. DIRECT SEROTONIN
11.3.1.3.1. TEGASEROD (ZELNORM)
11.3.1.3.1.1 MARKET SHARE (USD)
11.3.1.3.1.2 MARKET VOLUME (UNIT)
11.3.1.3.1.3 AVERAGE SELLING PRICE (USD)
11.3.1.3.2. ALOSETRON (LOTRONEX)
11.3.1.3.2.1 MARKET SHARE (USD)
11.3.1.3.2.2 MARKET VOLUME (UNIT)
11.3.1.3.2.3 AVERAGE SELLING PRICE (USD)
11.3.1.4. ANTI-DIARRHEAL
11.3.1.4.1. BY TYPE
11.3.1.4.1.1 ELUXADOLINE
11.3.1.4.1.1.1. MARKET SHARE (USD)
11.3.1.4.1.1.2. MARKET VOLUME (UNIT)
11.3.1.4.1.1.3. AVERAGE SELLING PRICE (USD)
11.3.1.4.1.2 DIPHENOXYLATE
11.3.1.4.1.2.1. MARKET SHARE (USD)
11.3.1.4.1.2.2. MARKET VOLUME (UNIT)
11.3.1.4.1.2.3. AVERAGE SELLING PRICE (USD)
11.3.1.4.1.3 LOPERAMIDE
11.3.1.4.1.3.1. MARKET SHARE (USD)
11.3.1.4.1.3.2. MARKET VOLUME (UNIT)
11.3.1.4.1.3.3. AVERAGE SELLING PRICE (USD)
11.3.1.4.1.4 CODEINE
11.3.1.4.1.4.1. MARKET SHARE (USD)
11.3.1.4.1.4.2. MARKET VOLUME (UNIT)
11.3.1.4.1.4.3. AVERAGE SELLING PRICE (USD)
11.3.1.4.1.5 OTHERS
11.3.1.4.2. BY DRUG TYPE
11.3.1.4.2.1 GENERIC
11.3.1.4.2.2 BRANDED
11.3.1.4.2.2.1. IMODIUM
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.4.2.2.2. VIBERZI
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.4.2.2.3. LOMOTIL
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.4.2.2.4. OTHERS
11.3.1.5. ANTIDEPRESSANTS
11.3.1.5.1. TRICYCLIC ANTIDEPRESSANTS
11.3.1.5.1.1 BY TYPE
11.3.1.5.1.1.1. IMIPRAMINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.1.1.2. DESIPRAMINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.1.1.3. NORTRIPTYLINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.1.1.4. OTHERS
11.3.1.5.1.2 BY DRUG TYPE
11.3.1.5.1.2.1. GENERIC
11.3.1.5.1.2.2. BRANDED
11.3.1.5.1.2.3. TOFRANIL
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.1.2.4. NORPRAMIN
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.1.2.5. PAMELOR
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.1.2.6. OTHERS
11.3.1.5.2. SSRI ANTIDEPRESSANTS
11.3.1.5.2.1 BY TYPE
11.3.1.5.2.1.1. FLUOXETINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.2.1.2. PAROXETINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.2.1.3. OTHERS
11.3.1.5.2.2 BY DRUG TYPE
11.3.1.5.2.2.1. GENERIC
11.3.1.5.2.2.2. BRANDED
11.3.1.5.2.2.3. PROZAC
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.2.2.4. PAXIL
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.5.2.2.5. OTHERS
11.3.1.6. ANTIBIOTICS
11.3.1.6.1. BY TYPE
11.3.1.6.1.1 RIFAXIMIN
11.3.1.6.1.1.1. MARKET SHARE (USD)
11.3.1.6.1.1.2. MARKET VOLUME (UNIT)
11.3.1.6.1.1.3. AVERAGE SELLING PRICE (USD)
11.3.1.6.1.2 NEOMYCIN
11.3.1.6.1.2.1. MARKET SHARE (USD)
11.3.1.6.1.2.2. MARKET VOLUME (UNIT)
11.3.1.6.1.2.3. AVERAGE SELLING PRICE (USD)
11.3.1.6.1.3 METRONIDAZOLE
11.3.1.6.1.3.1. MARKET SHARE (USD)
11.3.1.6.1.3.2. MARKET VOLUME (UNIT)
11.3.1.6.1.3.3. AVERAGE SELLING PRICE (USD)
11.3.1.6.1.4 CLARITHROMYCIN
11.3.1.6.1.4.1. MARKET SHARE (USD)
11.3.1.6.1.4.2. MARKET VOLUME (UNIT)
11.3.1.6.1.4.3. AVERAGE SELLING PRICE (USD)
11.3.1.6.1.5 OTHERS
11.3.1.6.1.6 BY DRUG TYPE
11.3.1.6.1.7 GENERIC
11.3.1.6.1.8 BRANDED
11.3.1.6.1.8.1. XIFAXAN
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.6.1.8.2. BIAXIN
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.6.1.8.3. FLAGYL
11.3.1.6.1.8.4. OTHERS
11.3.1.7. ANTISPASMODICS
11.3.1.7.1. BY TYPE
11.3.1.7.1.1 ANTIMUSCARINICS
11.3.1.7.1.1.1. DICYCLOVERINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.1.2. HYOSCINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.1.3. ATROPINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.1.4. PROPANTHELINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.2 ANTIMUSCARINICS
11.3.1.7.1.2.1. DICYCLOVERINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.2.2. HYOSCINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.2.3. ATROPINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.7.1.2.4. PROPANTHELINE
A. MARKET SHARE (USD)
B. MARKET VOLUME (UNIT)
C. AVERAGE SELLING PRICE (USD)
11.3.1.8. OTHERS
11.3.2 THERAPY
11.3.2.1. COGNITIVE BEHAVIOURAL THERAPY
11.3.2.2. GUT-DIRECTED HYPNOTHERAPY
11.3.2.3. RELAXATION TRAINING
11.3.2.4. OTHERS
12 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY DOSAGE
12.1 OVERVIEW
12.2 ORAL
12.2.1 SOLID
12.2.1.1. TABLETS
12.2.1.2. CAPSULES
12.2.1.3. POWDER
12.2.1.4. PILLS
12.2.1.5. OTHERS
12.2.2 SEMI-SOLID
12.2.2.1. GELS
12.2.2.2. EMULSIONS
12.2.2.3. ELIXIRS
12.2.2.4. OTHERS
12.2.3 LIQUID
12.2.3.1. SOLUTIONS
12.2.3.2. SYRUPS
12.2.3.3. OTHERS
12.3 PARENTERAL
12.3.1 CONVENTIONAL DRUGS DELIVERY FORMULATIONS
12.3.1.1. SOLUTIONS
12.3.1.2. RECONSTITUTED/LYOPHILIZED
12.3.1.3. SUSPENSIONS
12.3.1.4. EMULSIONS
12.3.1.5. OTHERS
12.3.2 NOVEL DRUGS DELIVERY FORMULATIONS
12.3.2.1. COLLOIDAL DISPERSIONS
12.3.2.2. MICROPARTICLES
12.3.2.3. LONG ACTING INJECTION FORMULATION
12.4 OTHERS
13 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY PATIENT TYPE
13.1 OVERVIEW
13.2 CHILD
13.2.1 MALE
13.2.2 FEMALE
13.3 ADULT
13.3.1 MALE
13.3.2 FEMALE
13.4 GERIATRIC
13.4.1 MALE
13.4.2 FEMALE
14 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITAL
14.3 CLINICS
14.4 HOME HEALTHCARE
14.5 SPECIALITY CENTER
14.6 AMBULTORY CENTERS
14.7 OTHERS
15 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 HOSPITAL PHARMACY
15.3 ONLINE PHARMACY
15.4 RETAIL PHARMACY
15.5 OTHERS
16 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY GEOGRAPHY
16.1 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
16.2 NORTH AMERICA
16.2.1 U.S.
16.2.1.1. U.S. IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY TYPE
16.2.1.2. U.S. IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY DIAGNOSIS & TREATMENT
16.2.1.3. U.S. IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY DOSAGE
16.2.1.4. U.S. IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY PATIENT TYPE
16.2.1.5. U.S. IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY END USER
16.2.1.6. U.S. IRRITABLE BOWEL SYNDROME (IBS) MARKET, BY DISTRIBUTION CHANNEL
16.2.2 CANADA
16.2.3 MEXICO
16.3 EUROPE
16.3.1 GERMANY
16.3.2 FRANCE
16.3.3 U.K.
16.3.4 HUNGARY
16.3.5 LITHUANIA
16.3.6 AUSTRIA
16.3.7 IRELAND
16.3.8 NORWAY
16.3.9 POLAND
16.3.10 ITALY
16.3.11 SPAIN
16.3.12 RUSSIA
16.3.13 TURKEY
16.3.14 NETHERLANDS
16.3.15 SWITZERLAND
16.3.16 REST OF EUROPE
16.4 ASIA-PACIFIC
16.4.1 JAPAN
16.4.2 CHINA
16.4.3 SOUTH KOREA
16.4.4 INDIA
16.4.5 AUSTRALIA
16.4.6 SINGAPORE
16.4.7 THAILAND
16.4.8 MALAYSIA
16.4.9 INDONESIA
16.4.10 PHILIPPINES
16.4.11 VIETNAM
16.4.12 REST OF ASIA-PACIFIC
16.5 SOUTH AMERICA
16.5.1 BRAZIL
16.5.2 ARGENTINA
16.5.3 PERU
16.5.4 COLOMBIA
16.5.5 REST OF SOUTH AMERICA
16.6 MIDDLE EAST AND AFRICA
16.6.1 SOUTH AFRICA
16.6.2 SAUDI ARABIA
16.6.3 UAE
16.6.4 EGYPT
16.6.5 KUWAIT
16.6.6 ISRAEL
16.6.7 REST OF MIDDLE EAST AND AFRICA
16.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
17 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, SWOT AND DBMR ANALYSIS
18 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: GLOBAL
18.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
18.3 COMPANY SHARE ANALYSIS: EUROPE
18.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.5 MERGERS & ACQUISITIONS
18.6 NEW PRODUCT DEVELOPMENT & APPROVALS
18.7 EXPANSIONS
18.8 REGULATORY CHANGES
18.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
19 GLOBAL IRRITABLE BOWEL SYNDROME (IBS) MARKET, COMPANY PROFILE
19.1 VALEANT PHARMACEUTICALS INTERNATIONAL INC
19.1.1 COMPANY OVERVIEW
19.1.2 REVENUE ANALYSIS
19.1.3 GEOGRAPHIC PRESENCE
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENTS
19.2 NOVARTIS AG
19.2.1 COMPANY OVERVIEW
19.2.2 REVENUE ANALYSIS
19.2.3 GEOGRAPHIC PRESENCE
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 SUCAMPO PHARMACEUTICALS, INC
19.3.1 COMPANY OVERVIEW
19.3.2 REVENUE ANALYSIS
19.3.3 GEOGRAPHIC PRESENCE
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.4 TAKEDA PHARMACEUTICAL COMPANY LIMITED
19.4.1 COMPANY OVERVIEW
19.4.2 REVENUE ANALYSIS
19.4.3 GEOGRAPHIC PRESENCE
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENTS
19.5 JOHNSON & JOHNSON SERVICES INC
19.5.1 COMPANY OVERVIEW
19.5.2 REVENUE ANALYSIS
19.5.3 GEOGRAPHIC PRESENCE
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENTS
19.6 LEXICON PHARMACEUTICALS
19.6.1 COMPANY OVERVIEW
19.6.2 REVENUE ANALYSIS
19.6.3 GEOGRAPHIC PRESENCE
19.6.4 PRODUCT PORTFOLIO
19.6.5 RECENT DEVELOPMENTS
19.7 GLAXOSMITHKLINE PLC
19.7.1 COMPANY OVERVIEW
19.7.2 REVENUE ANALYSIS
19.7.3 GEOGRAPHIC PRESENCE
19.7.4 PRODUCT PORTFOLIO
19.7.5 RECENT DEVELOPMENTS
19.8 BAYER AG
19.8.1 COMPANY OVERVIEW
19.8.2 REVENUE ANALYSIS
19.8.3 GEOGRAPHIC PRESENCE
19.8.4 PRODUCT PORTFOLIO
19.8.5 RECENT DEVELOPMENTS
19.9 ALLERGEN PLC
19.9.1 COMPANY OVERVIEW
19.9.2 REVENUE ANALYSIS
19.9.3 GEOGRAPHIC PRESENCE
19.9.4 PRODUCT PORTFOLIO
19.9.5 RECENT DEVELOPMENTS
19.1 MYLAN N.V.
19.10.1 COMPANY OVERVIEW
19.10.2 REVENUE ANALYSIS
19.10.3 GEOGRAPHIC PRESENCE
19.10.4 PRODUCT PORTFOLIO
19.10.5 RECENT DEVELOPMENTS
19.11 REDHILL
19.11.1 COMPANY OVERVIEW
19.11.2 REVENUE ANALYSIS
19.11.3 GEOGRAPHIC PRESENCE
19.11.4 PRODUCT PORTFOLIO
19.11.5 RECENT DEVELOPMENTS
19.12 PFIZER
19.12.1 COMPANY OVERVIEW
19.12.2 REVENUE ANALYSIS
19.12.3 GEOGRAPHIC PRESENCE
19.12.4 PRODUCT PORTFOLIO
19.12.5 RECENT DEVELOPMENTS
19.13 BRISTOL-MYERS SQUIBB COMPANY
19.13.1 COMPANY OVERVIEW
19.13.2 REVENUE ANALYSIS
19.13.3 GEOGRAPHIC PRESENCE
19.13.4 PRODUCT PORTFOLIO
19.13.5 RECENT DEVELOPMENTS
19.14 SUN PHARMACEUTICAL INDUSTRIES LTD
19.14.1 COMPANY OVERVIEW
19.14.2 REVENUE ANALYSIS
19.14.3 GEOGRAPHIC PRESENCE
19.14.4 PRODUCT PORTFOLIO
19.14.5 RECENT DEVELOPMENTS
19.15 SLOAN PHARMACEUTICALS, INC
19.15.1 COMPANY OVERVIEW
19.15.2 REVENUE ANALYSIS
19.15.3 GEOGRAPHIC PRESENCE
19.15.4 PRODUCT PORTFOLIO
19.15.5 RECENT DEVELOPMENTS
19.16 JAGUAR HEALTH
19.16.1 COMPANY OVERVIEW
19.16.2 REVENUE ANALYSIS
19.16.3 GEOGRAPHIC PRESENCE
19.16.4 PRODUCT PORTFOLIO
19.16.5 RECENT DEVELOPMENTS
19.17 IRONWOOD PHARMACEUTICALS, INC
19.17.1 COMPANY OVERVIEW
19.17.2 REVENUE ANALYSIS
19.17.3 GEOGRAPHIC PRESENCE
19.17.4 PRODUCT PORTFOLIO
19.17.5 RECENT DEVELOPMENTS
19.18 SUMITOMO DAINIPPON PHARMA LTD
19.18.1 COMPANY OVERVIEW
19.18.2 PRODUCT PORTFOLIO
19.18.3 REVENUE ANALYSIS
19.18.4 GEOGRAPHIC PRESENCE
19.18.5 PRODUCT PORTFOLIO
19.19 ASTRAZENECA
19.19.1 COMPANY OVERVIEW
19.19.2 PRODUCT PORTFOLIO
19.19.3 REVENUE ANALYSIS
19.19.4 GEOGRAPHIC PRESENCE
19.19.5 PRODUCT PORTFOLIO
19.2 SYNTHETIC BIOLOGICS
19.20.1 COMPANY OVERVIEW
19.20.2 PRODUCT PORTFOLIO
19.20.3 REVENUE ANALYSIS
19.20.4 GEOGRAPHIC PRESENCE
19.20.5 PRODUCT PORTFOLIO
19.21 ARDELYX
19.21.1 COMPANY OVERVIEW
19.21.2 PRODUCT PORTFOLIO
19.21.3 REVENUE ANALYSIS
19.21.4 GEOGRAPHIC PRESENCE
19.21.5 PRODUCT PORTFOLIO
19.22 ASTELLAS
19.22.1 COMPANY OVERVIEW
19.22.2 PRODUCT PORTFOLIO
19.22.3 REVENUE ANALYSIS
19.22.4 GEOGRAPHIC PRESENCE
19.22.5 PRODUCT PORTFOLIO
19.23 THE MENARINI GROUP
19.23.1 COMPANY OVERVIEW
19.23.2 PRODUCT PORTFOLIO
19.23.3 REVENUE ANALYSIS
19.23.4 GEOGRAPHIC PRESENCE
19.23.5 PRODUCT PORTFOLIO
19.24 AUROBINDO PHARMA USA
19.24.1 COMPANY OVERVIEW
19.24.2 PRODUCT PORTFOLIO
19.24.3 REVENUE ANALYSIS
19.24.4 GEOGRAPHIC PRESENCE
19.24.5 PRODUCT PORTFOLIO
19.25 SUN PHARMACEUTICAL INDUSTRIES, INC.
19.25.1 COMPANY OVERVIEW
19.25.2 PRODUCT PORTFOLIO
19.25.3 REVENUE ANALYSIS
19.25.4 GEOGRAPHIC PRESENCE
19.25.5 PRODUCT PORTFOLIO
19.26 JOHNSON & JOHNSON SERVICES, INC.
19.26.1 COMPANY OVERVIEW
19.26.2 PRODUCT PORTFOLIO
19.26.3 REVENUE ANALYSIS
19.26.4 GEOGRAPHIC PRESENCE
19.26.5 PRODUCT PORTFOLIO
19.27 BIOAMERICA
19.27.1 COMPANY OVERVIEW
19.27.2 PRODUCT PORTFOLIO
19.27.3 REVENUE ANALYSIS
19.27.4 GEOGRAPHIC PRESENCE
19.27.5 PRODUCT PORTFOLIO
19.28 EVOGENE
19.28.1 COMPANY OVERVIEW
19.28.2 PRODUCT PORTFOLIO
19.28.3 REVENUE ANALYSIS
19.28.4 GEOGRAPHIC PRESENCE
19.28.5 PRODUCT PORTFOLIO
19.29 4D PHARMA PLC
19.29.1 COMPANY OVERVIEW
19.29.2 PRODUCT PORTFOLIO
19.29.3 REVENUE ANALYSIS
19.29.4 GEOGRAPHIC PRESENCE
19.29.5 PRODUCT PORTFOLIO
19.3 SHIELD THERAPEUTICS
19.30.1 COMPANY OVERVIEW
19.30.2 PRODUCT PORTFOLIO
19.30.3 REVENUE ANALYSIS
19.30.4 GEOGRAPHIC PRESENCE
19.30.5 PRODUCT PORTFOLIO
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
20 RELATED REPORTS
21 CONCLUSION
22 QUESTIONNAIRE
23 ABOUT DATA BRIDGE MARKET RESEARCH
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。