Global Molecular Diagnostics Market
市场规模(十亿美元)
CAGR :
% 
 USD
25.80 Billion
USD
70.05 Billion
2024
2032
USD
25.80 Billion
USD
70.05 Billion
2024
2032
| 2025 –2032 | |
| USD 25.80 Billion | |
| USD 70.05 Billion | |
|
|
|
|
全球分子診斷市場細分,按產品(試劑和試劑盒、儀器、服務和軟體)、技術(質譜 (MS)、毛細管電泳、下一代定序 (NGS)、晶片和微陣列、基於聚合酶鍊式反應 (PCR) 的方法、細胞遺傳學、原位雜交 (ISH 或FISH)、分子影像及其他)、應用(腫瘤學、藥物基因組學、微生物學、產前檢測、組織分型、血液篩檢、心血管疾病、神經系統疾病、傳染病及其他)和最終用戶(醫院、臨床實驗室和學術機構)劃分——產業趨勢及至 2032 年的預測
分子診斷市場規模
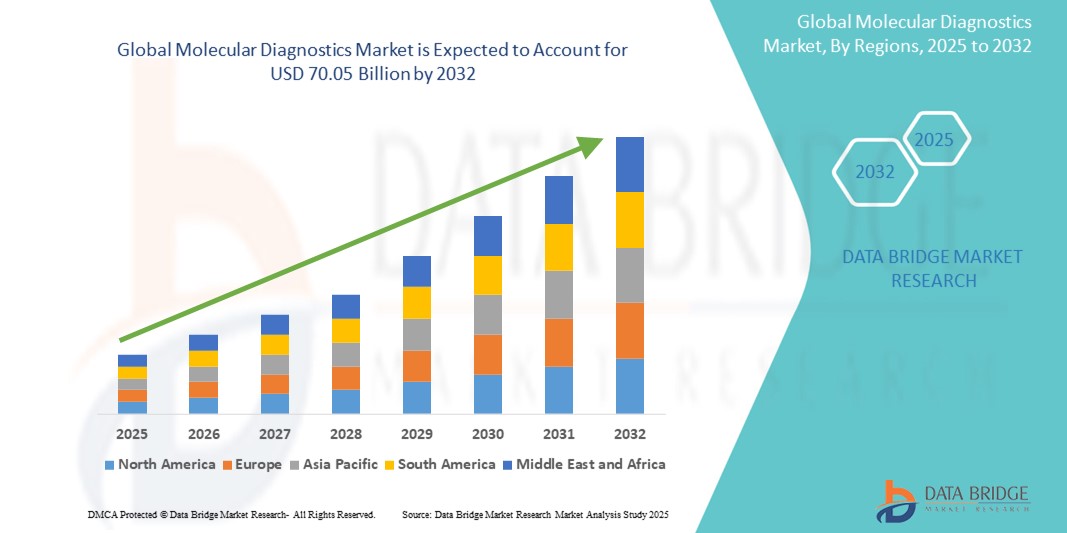
- 2024年全球分子診斷市場價值為258億美元,預計2032年將達到700.5億美元。
- 在2025年至2032年的預測期內,該市場預計將以13.30%的複合年增長率成長,主要驅動力是PCR的高使用量。
- 這種成長是由準確性、靈敏度和快速週轉時間等因素所驅動的。
分子診斷市場分析
- 分子診斷是指利用分子生物學技術(例如PCR和NGS)來檢測和監測疾病、識別遺傳易感性並指導標靶治療的醫學檢測領域,廣泛應用於醫療保健、臨床實驗室和研究機構。
- 市場成長的驅動因素包括傳染病和慢性病盛行率的上升、對個人化醫療需求的增加以及即時PCR和下一代定序等先進診斷技術的日益普及。
- 隨著自動化平台的技術創新、人工智慧和機器學習在結果解讀中的應用,以及便攜式即時診斷設備的開發,市場正在不斷發展,這些創新提高了診斷的速度、準確性和便利性。
- 例如,羅氏和賽默飛世爾科技等公司正在利用數位技術和自動化手段,提供更快、更精準的分子診斷解決方案,以滿足臨床和實驗室的需求。
- 分子診斷市場預計將持續成長,這得益於基因組學的不斷進步、醫療基礎設施的不斷完善以及對疾病早期檢測和精準醫療項目投資的增加。
報告範圍和分子診斷市場細分
|
屬性 |
分子診斷關鍵市場洞察 |
|
涵蓋部分 |
|
|
覆蓋國家/地區 |
北美洲
歐洲
亞太
中東和非洲
南美洲
|
|
主要市場參與者 |
|
|
市場機遇 |
|
|
加值資料資訊集 |
除了對市場狀況(如市場價值、成長率、細分、地理覆蓋範圍和主要參與者)的洞察之外,Data Bridge Market Research 精心編制的市場報告還包括深入的專家分析、患者流行病學、產品線分析、定價分析和監管框架。 |
分子診斷市場趨勢
“液體活檢技術的應用日益普及”
- 全球分子診斷市場的一個顯著趨勢是液體活檢技術的日益普及。
- 這一趨勢是由對非侵入性診斷方法日益增長的需求、對疾病進展進行即時監測的需要以及對個人化治療策略(尤其是在腫瘤學領域)日益重視所驅動的。
- 例如,Guardant Health 和 Biocept 等公司正在推進液體活檢技術的發展,該技術透過分析循環腫瘤 DNA (ctDNA) 和其他生物標誌物,實現癌症的早期檢測和治療方案的選擇。
- 隨著微創手術的不斷普及,以及生物標記檢測和基因組分析技術的進步,液體活檢正在加速融入常規臨床實踐。
- 隨著醫療保健系統日益重視精準醫療、早期診斷和改善患者預後,液體活檢有望在擴大各種疾病領域及時、準確和個性化診斷的可及性方面發揮變革性作用。
分子診斷市場動態
司機
“老年人口不斷增加”
- 人口老化加劇是全球分子診斷市場成長的關鍵驅動因素。
- 這種人口結構的變化導致癌症、心血管疾病和神經退化性疾病等與年齡相關的疾病發生率上升,從而推動了對早期準確診斷解決方案的需求。
- 隨著人口老化,人們對健康監測和個人化照護的需求日益增長,分子診斷技術提供了一種非侵入性、快速、精準的檢測方法,適用於慢性病管理和預防保健。
- 分子診斷具有早期檢測、標靶治療指導和即時疾病追蹤等優勢,使其成為整個醫療保健系統中老年護理策略的重要工具。
- 診斷解決方案提供者正在積極應對,開發出使用者友好、微創的平台,以滿足老年患者的需求,並透過居家檢測和遠距醫療整合來擴大服務範圍。
例如,
- 羅氏和雅培正在推動分子檢測工具的研發,用於癌症和傳染病的早期發現,尤其關注老年人的診斷需求。
- 賽默飛世爾科技正在增強其基因組平台,以支持針對年齡相關遺傳風險的個人化診斷。
- 隨著全球人口老化加劇,醫療保健重點轉向預防和個人化護理,分子診斷在支持老年人長期健康結果方面的作用預計將顯著擴大。
機會
“即時檢測中對分子診斷的需求日益增長”
- 即時檢測(POC)環境下對分子診斷日益增長的需求,為分子診斷市場帶來了巨大的機會。 POC診斷能夠實現快速的現場檢測,從而提升了檢測的可及性、速度和即時臨床決策能力。
- 便攜式技術的進步、用戶友好的檢測方法以及整合的樣本到結果系統的出現,使醫療保健提供者能夠在診所、急診室和偏遠地區等場所提供即時診斷資訊。
- 緊湊的設計、極少的樣本需求和自動化工作流程等特點正被用於支援更快的診斷和治療啟動,尤其是在傳染病管理和慢性病監測方面。
例如,
- Cepheid公司的GeneXpert平台和Abbott公司的ID NOW平台廣泛應用於分散式醫療環境中,用於快速、準確的分子檢測。
- 生物梅里埃公司正在開發緊湊型分子系統,旨在簡化急診護理和資源匱乏環境下的診斷流程。
- 隨著全球醫療保健系統努力改善患者預後、減少診斷延誤並擴大優質醫療服務的覆蓋範圍,即時分子診斷技術的持續發展有望在已開發市場和新興市場都帶來新的機會。
克制/挑戰
“增加監管審批”
- 日益增多的監管審批和合規要求給分子診斷市場帶來了巨大挑戰。雖然審批對於確保安全性和有效性至關重要,但全球監管流程的複雜性和差異性可能會延緩產品開發和市場准入。
- 對於希望在多個地區推出創新診斷技術的公司而言,這項挑戰尤其突出,因為他們必須應對不同的標準、文件和審批時間表。
- 冗長的審批流程可能會延遲關鍵診斷工具的上市,增加研發成本,並為市場上規模較小或新興的企業設置障礙。
例如,
- 新創公司在尋求新型分子檢測方法的FDA或CE認證時,往往面臨較長的審批時間,可能會阻礙其商業化策略。
- 如果缺乏簡化的監管途徑和國際協調,分子診斷市場可能會面臨創新成果推廣延遲的問題,從而限制患者及時獲得先進診斷技術,並影響患者的治療效果。
分子診斷市場範圍
市場按產品、技術、應用和最終用戶進行細分。
|
分割 |
子細分 |
|
副產品 |
|
|
透過技術 |
|
|
透過申請 |
|
|
最終用戶
|
|
分子診斷市場區域分析
“北美是分子診斷市場的主導地區”
- 北美在分子診斷市場佔據主導地位,這主要得益於醫療保健支出增加、醫療設施先進以及完善的醫療保健體系,這些因素共同支持創新和醫療服務的可及性。
- 美國佔了相當大的份額,這得益於其高人均醫療保健支出、大量的研究機構和生物技術公司,以及消費者對早期疾病檢測和個人化醫療重要性的廣泛認識。
- 北美領導者持續創新,開發新一代診斷平台,整合人工智慧、分子生物學進展和雲端技術。這帶來了更有效率、可擴展且更精準的診斷解決方案,從而改善患者預後和醫療服務品質。
- 憑藉強大的基礎設施、老化的人口以及消費者對精準醫療日益增長的需求,預計北美將在2025年至2032年的預測期內保持其作為分子診斷領域規模最大、技術最先進的市場的地位。
“亞太地區預計將實現最高成長率”
- 受人口結構快速變化、人口老化以及癌症和傳染病等疾病日益普遍的推動,亞太地區預計將成為分子診斷市場成長最快的地區。
- 中國、印度和日本等國憑藉其不斷完善的醫療基礎設施、政府為改善醫療服務可及性而採取的舉措以及人們對疾病早期檢測意識的提高,正引領著區域增長。
- 該地區在醫療保健技術領域也取得了顯著進步,包括遠距醫療、基因組學和行動健康診斷,這些進步推動了對能夠處理大量患者並提供準確、經濟高效檢測的先進分子診斷工具的需求。
- 隨著醫療數位化程度的不斷提高、政府對生物技術進步的支持以及個人化醫療的日益普及,亞太地區已成為分子診斷領域中成長最快的區域市場。隨著醫療體系的不斷發展,亞太地區在2025年至2032年間將持續擴大市場份額,成為分子診斷創新和應用的重要中心。
分子診斷市佔率
市場競爭格局部分按競爭對手提供詳細信息,包括公司概況、財務狀況、收入、市場潛力、研發投入、新市場拓展計劃、全球佈局、生產基地及設施、產能、公司優勢與劣勢、產品發布、產品線寬度與廣度以及應用領域優勢。以上數據僅與各公司在市場上的業務重點相關。
市場上的主要市場領導者包括:
- F. Hoffmann-La Roche Ltd(瑞士)
- Hologic公司(美國)
- 生物梅里埃公司(法國)
- 雅培實驗室(美國)
- QIAGEN NV(荷蘭)
- 賽默飛世爾科技公司(美國)
- 西門子股份公司(德國)
- 丹納赫公司(美國)
- Myriad Genetics, Inc.(美國)
- Illumina公司(美國)
- 安捷倫科技公司(美國)
- 貝克頓·迪金森公司(BD)(美國)
- 迪亞索林公司(義大利)
- 格里福爾斯公司(西班牙)
- QuidelOrtho公司(美國)
- Genetic Signatures Limited(澳洲)
- MDxHealth SA(比利時)
- 精確科學公司(美國)
- Biocartis Group NV(比利時)
- TBG Diagnostics Limited(澳洲)
- GenMark Diagnostics, Inc.(美國)
- Luminex公司(美國)
- HTG分子診斷公司(美國)
- Vela Diagnostics(新加坡)
- 廈門艾德生物診斷股份有限公司(中國)
- Molbio Diagnostics Pvt. Ltd.(印度)
- geneOmbio Technologies Pvt. Ltd.(印度)
全球分子診斷市場最新發展
- 2021年,羅氏完成了對TIB Molbiol集團的收購。 TIB Molbiol集團擁有約45項獲得CE-IVD認證的檢測方法,用於診斷遺傳性疾病、傳染病、移植疾病和血液學疾病。
- 2020年,羅氏診斷印度公司在加爾各答國家霍亂和腸道疾病研究所推出了Cobas 8800和Cobas 6800系統,以輔助SARS-CoV-2的診斷檢測。羅氏Cobas 6800/8800系統可在三個半小時內提供檢測結果,並可顯著提高操作效率、靈活性,以及實現最快的出結果速度。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MOLECULAR DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MOLECULAR DIAGNOSTICS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MOLECULAR DIAGNOSTICS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER'S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNOLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT AND SERVICES
17.1 OVERVIEW
17.2 PRODUCT
17.2.1 INSTRUMENTS
17.2.1.1. BY TYPE
17.2.1.1.1. FULLY AUTOMATED
17.2.1.1.2. SEMI-AUTOMATED
17.2.1.1.3. MANUAL
17.2.1.2. BY MODALITY
17.2.1.2.1. PORTABLE
17.2.1.2.2. STANDALONE
17.2.1.2.3. BENCHTOP
17.2.2 REAGENTS AND KITS
17.2.2.1. KITS
17.2.2.1.1. DNA EXTRACTION AND PURIFICATION KITS
17.2.2.1.1.1 PLASMID MINIPREP AND MAXIPREP KITS
17.2.2.1.1.2 PLASMID PURIFICATION KITS
17.2.2.1.1.3 PCR PURIFICATION KITS
17.2.2.1.1.4 GENOMIC DNA PURIFICATION KITS
17.2.2.1.1.5 OTHERS
17.2.2.1.2. RNAI AND RNA REAGENTS
17.2.2.1.3. NUCLEIC ACID PURIFICATION KITS
17.2.2.1.4. NUCLEIC ACID SYNTHESIS KITS, BUFFERS & REAGENTS
17.2.2.1.5. HPV KITS
17.2.2.1.6. TB KITS
17.2.2.1.7. DEPLETION KITS
17.2.2.1.8. OTHERS
17.2.2.2. QC SETS AND PANELS
17.2.2.2.1. CONTROL PANEL
17.2.2.2.1.1 MULTIPLEX VAGINAL CONTROL PANEL
17.2.2.2.1.2 HUMAN PAPILLOMAVIRUS (HPV) CONTROL PANEL
17.2.2.2.1.3 CANDIDA VAGINITIS/TRICHOMONAS VAGINALIS (CV/TV) CONTROL PANEL
17.2.2.2.1.4 CT/NG CONTROL PANEL
17.2.2.2.1.5 BACTERIAL VAGINOSIS (BV) CONTROL PANEL
17.2.2.2.1.6 CELLULARITY CONTROL
17.2.2.2.1.7 RESPIRATORY CONTROL PANEL
17.2.2.2.1.8 FLU/RSV/SARS-COV-2 CONTROL PANEL
17.2.2.2.1.8.1. NXG CONTROL PANEL
17.2.2.2.1.8.2. COMPLETE CONTROL PANEL
17.2.2.2.1.8.3. OTHERS
17.2.2.2.1.9 MPN PANEL
17.2.2.2.1.10 AML PANEL
17.2.2.2.1.11 QC PANEL
17.2.2.2.1.12 ALL PANEL
17.2.2.2.1.13 VERIFICATION PANEL
17.2.2.2.1.14 OTHERS
17.2.2.3. MUTATIONS DETECTION KIT
17.2.2.3.1. KRAS PCR KIT
17.2.2.3.2. EGFR KIT
17.2.2.3.3. BRAF MUTATION KIT
17.2.2.3.4. AML1-ETO KIT
17.2.2.3.5. NRAS MUTATION KIT
17.2.2.3.6. CALR KIT
17.2.2.3.7. FLT3 MUTATION DETECTION
17.2.2.3.8. C-KIT MUTATION DETECTION KIT
17.2.2.3.9. MGMT METHYLATION DETECTION KIT
17.2.2.3.10. CBFB-MYH11 KIT
17.2.2.3.11. GENE FUSIONS DETECTION KIT
17.2.2.3.12. OTHER KITS AND ASSAYS
17.2.2.4. ENZYMES
17.2.2.4.1. POLYMERASES
17.2.2.4.2. LIGASES
17.2.2.4.3. RESTRICTION ENDONUCLEASES
17.2.2.4.4. REVERSE TRANSCRIPTASES
17.2.2.4.5. PHOSPHATASES
17.2.2.4.6. PROTEASES AND PROTEINASES
17.2.2.4.7. DNA LADDERS
17.2.2.4.8. OTHER ENZYMES
17.2.2.5. BUFFERS
17.2.2.6. PRIMERS
17.2.2.7. OTHERS
17.3 SOFTWARE AND SERVICES
17.3.1 SERVICES
17.3.1.1. INSTRUMENT REPAIR SERVICES
17.3.1.1.1. ON-SITE REPAIR SERVICES
17.3.1.1.2. OFF-SITE REPAIR SERVICES
17.3.1.2. TRAINING SERVICES
17.3.1.2.1. PCR BASED MOLECULAR DIAGNOSTICS
17.3.1.2.2. IMMUNOHISTOCHEMISTRY (IHC)
17.3.1.2.3. VIRAL LOAD TESTING
17.3.1.2.4. INTERPHASE CHROMOSOME PROFILING (ICP)
17.3.1.2.5. OTHERS
17.3.1.3. COMPLIANCE SERVICES
17.3.1.3.1. IQ/OQ & PM/OQ SERVICES
17.3.1.3.2. INSTALLATION SERVICES
17.3.1.3.3. VALIDATION SERVICES
17.3.1.3.4. OTHER SERVICES
17.3.1.4. SCALABLE AUTOMATION SERVICES
17.3.1.4.1. AUTOMATION FOR HIGH-CONTENT SCREENING (HCS)
17.3.1.4.2. AUTOMATION FOR HIGH-THROUGHPUT PLATE-BASED ASSAYS
17.3.1.4.3. AUTOMATION FOR HIGH-THROUGHPUT CLONE SCREENING
17.3.1.5. CALIBRATION SERVICES
17.3.1.6. MAINTENANCE SERVICES
17.3.1.7. INSTRUMENT RELOCATION SERVICES
17.3.1.7.1. PLATE RECERTIFICATION
17.3.1.7.2. FACTORY APPROVED PARTS
17.3.1.7.3. INSTRUMENT PERFORMANCE EVALUATION
17.3.1.7.4. OTHER SERVICES
17.3.1.8. HARDWARE CUSTOMIZATION
17.3.1.9. PERFORMANCE ASSURANCE SERVICES
17.3.1.10. DESIGN AND DEVELOPMENT SERVICES
17.3.1.11. SUPPLY CHAIN SOLUTIONS
17.3.1.12. CLINICAL RESEARCH SERVICES
17.3.1.13. OTHER SERVICES
17.3.2 SOFTWARE
17.3.2.1. BY TYPE
17.3.2.1.1. INTEGRATED
17.3.2.1.2. STANDALONE
17.3.2.2. BY DEPLOYMENT
17.3.2.2.1. CLOUD BASES
17.3.2.2.2. ON-PREMISES
17.3.2.2.3. HYBRID
18 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNOLOGY
18.1 OVERVIEW
18.2 POLYMERASE CHAIN REACTION (PCR)
18.2.1 BY TYPE
18.2.1.1. REAL-TIME PCR
18.2.1.2. DIGITAL PCR
18.2.1.3. REVERSE TRANSCRIPTASE PCR
18.2.1.4. QUANTITATIVE FLUORESCENT PCR
18.2.1.5. COLD PCR
18.2.1.6. OTHERS
18.2.2 BY PRODUCT AND SERVICES
18.2.2.1. PRODUCT
18.2.2.2. SERVICES AND SOFTWARE
18.3 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
18.3.1 BY PRODUCT AND SERVICES
18.3.1.1. PRODUCT
18.3.1.2. SERVICES AND SOFTWARE
18.4 NEXT GENERATION SEQUENCING (NGS)
18.4.1 BY PRODUCT AND SERVICES
18.4.1.1. PRODUCT
18.4.1.2. SERVICES AND SOFTWARE
18.5 CYTOGENETICS
18.5.1 BY PRODUCT AND SERVICES
18.5.1.1. PRODUCT
18.5.1.2. SERVICES AND SOFTWARE
18.6 CAPILLARY ELECTROPHORESIS
18.6.1 BY PRODUCT AND SERVICES
18.6.1.1. PRODUCT
18.6.1.2. SERVICES AND SOFTWARE
18.7 IN SITU HYBRIDIZATION (ISH OR FISH)
18.7.1 BY PRODUCT AND SERVICES
18.7.1.1. PRODUCT
18.7.1.2. SERVICES AND SOFTWARE
18.8 MOLECULAR IMAGING
18.8.1 BY TYPE
18.8.1.1. OPTICAL IMAGING
18.8.1.2. FDG-PET
18.8.1.3. OTHERS
18.8.2 BY PRODUCT AND SERVICES
18.8.2.1. PRODUCT
18.8.2.2. SERVICES AND SOFTWARE
18.9 MASS SPECTROMETRY (MS)
18.9.1 BY PRODUCT AND SERVICES
18.9.1.1. PRODUCT
18.9.1.2. SERVICES AND SOFTWARE
18.1 CHIPS AND MICROARRAY
18.10.1 BY PRODUCT AND SERVICES
18.10.1.1. PRODUCT
18.10.1.2. SERVICES AND SOFTWARE
18.11 OTHERS
19 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 ONCOLOGY TESTING
19.2.1 ONCOLOGY, BY CANCER TYPE
19.2.1.1. BREAST CANCER
19.2.1.1.1. MARKET VALUE (USD MILLION)
19.2.1.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.1.3. AVERAGE TEST COST (USD)
19.2.1.2. COLORECTAL CANCER
19.2.1.2.1. MARKET VALUE (USD MILLION)
19.2.1.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.2.3. AVERAGE TEST COST (USD)
19.2.1.3. LUNG CANCER
19.2.1.3.1. MARKET VALUE (USD MILLION)
19.2.1.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.3.3. AVERAGE TEST COST (USD)
19.2.1.4. PROSTATE CANCER
19.2.1.4.1. MARKET VALUE (USD MILLION)
19.2.1.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.4.3. AVERAGE TEST COST (USD)
19.2.1.5. OTHERS
19.2.2 ONCOLOGY, BY TECHNOLOGY
19.2.2.1. POLYMERASE CHAIN REACTION (PCR)
19.2.2.1.1. MARKET VALUE (USD MILLION)
19.2.2.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.1.3. AVERAGE TEST COST (USD)
19.2.2.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.2.2.2.1. MARKET VALUE (USD MILLION)
19.2.2.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.2.3. AVERAGE TEST COST (USD)
19.2.2.3. NEXT GENERATION SEQUENCING (NGS)
19.2.2.3.1. MARKET VALUE (USD MILLION)
19.2.2.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.3.3. AVERAGE TEST COST (USD)
19.2.2.4. CYTOGENETICS
19.2.2.4.1. MARKET VALUE (USD MILLION)
19.2.2.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.4.3. AVERAGE TEST COST (USD)
19.2.2.5. CAPILLARY ELECTROPHORESIS
19.2.2.5.1. MARKET VALUE (USD MILLION)
19.2.2.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.5.3. AVERAGE TEST COST (USD)
19.2.2.6. IN SITU HYBRIDIZATION (ISH OR FISH)
19.2.2.6.1. MARKET VALUE (USD MILLION)
19.2.2.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.6.3. AVERAGE TEST COST (USD)
19.2.2.7. MOLECULAR IMAGING
19.2.2.7.1. MARKET VALUE (USD MILLION)
19.2.2.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.7.3. AVERAGE TEST COST (USD)
19.2.2.8. MASS SPECTROMETRY (MS)
19.2.2.8.1. MARKET VALUE (USD MILLION)
19.2.2.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.8.3. AVERAGE TEST COST (USD)
19.2.2.9. CHIPS AND MICROARRAY
19.2.2.9.1. MARKET VALUE (USD MILLION)
19.2.2.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.9.3. AVERAGE TEST COST (USD)
19.2.2.10. OTHERS
19.3 PHARMACOGENOMICS
19.3.1 POLYMERASE CHAIN REACTION (PCR)
19.3.1.1. MARKET VALUE (USD MILLION)
19.3.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.1.3. AVERAGE TEST COST (USD)
19.3.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.3.2.1. MARKET VALUE (USD MILLION)
19.3.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.2.3. AVERAGE TEST COST (USD)
19.3.3 NEXT GENERATION SEQUENCING (NGS)
19.3.3.1. MARKET VALUE (USD MILLION)
19.3.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.3.3. AVERAGE TEST COST (USD)
19.3.4 CYTOGENETICS
19.3.4.1. MARKET VALUE (USD MILLION)
19.3.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.4.3. AVERAGE TEST COST (USD)
19.3.5 CAPILLARY ELECTROPHORESIS
19.3.5.1. MARKET VALUE (USD MILLION)
19.3.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.5.3. AVERAGE TEST COST (USD)
19.3.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.3.6.1. MARKET VALUE (USD MILLION)
19.3.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.6.3. AVERAGE TEST COST (USD)
19.3.7 MOLECULAR IMAGING
19.3.7.1. MARKET VALUE (USD MILLION)
19.3.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.7.3. AVERAGE TEST COST (USD)
19.3.8 MASS SPECTROMETRY (MS)
19.3.8.1. MARKET VALUE (USD MILLION)
19.3.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.8.3. AVERAGE TEST COST (USD)
19.3.9 CHIPS AND MICROARRAY
19.3.9.1. MARKET VALUE (USD MILLION)
19.3.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.9.3. AVERAGE TEST COST (USD)
19.3.10 OTHERS
19.4 MICROBIOLOGY
19.4.1 POLYMERASE CHAIN REACTION (PCR)
19.4.1.1. MARKET VALUE (USD MILLION)
19.4.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.1.3. AVERAGE TEST COST (USD)
19.4.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.4.2.1. MARKET VALUE (USD MILLION)
19.4.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.2.3. AVERAGE TEST COST (USD)
19.4.3 NEXT GENERATION SEQUENCING (NGS)
19.4.3.1. MARKET VALUE (USD MILLION)
19.4.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.3.3. AVERAGE TEST COST (USD)
19.4.4 CYTOGENETICS
19.4.4.1. MARKET VALUE (USD MILLION)
19.4.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.4.3. AVERAGE TEST COST (USD)
19.4.5 CAPILLARY ELECTROPHORESIS
19.4.5.1. MARKET VALUE (USD MILLION)
19.4.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.5.3. AVERAGE TEST COST (USD)
19.4.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.4.6.1. MARKET VALUE (USD MILLION)
19.4.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.6.3. AVERAGE TEST COST (USD)
19.4.7 MOLECULAR IMAGING
19.4.7.1. MARKET VALUE (USD MILLION)
19.4.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.7.3. AVERAGE TEST COST (USD)
19.4.8 MASS SPECTROMETRY (MS)
19.4.8.1. MARKET VALUE (USD MILLION)
19.4.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.8.3. AVERAGE TEST COST (USD)
19.4.9 CHIPS AND MICROARRAY
19.4.9.1. MARKET VALUE (USD MILLION)
19.4.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.9.3. AVERAGE TEST COST (USD)
19.4.10 OTHERS
19.5 PRENATAL TESTS
19.5.1 SICKLE CELL DISEASE
19.5.1.1. MARKET VALUE (USD MILLION)
19.5.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.5.1.3. AVERAGE TEST COST (USD)
19.5.2 CYSTIC FIBROSIS
19.5.2.1. MARKET VALUE (USD MILLION)
19.5.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.5.2.3. AVERAGE TEST COST (USD)
19.5.3 TAY-SACHS DISEASE
19.5.3.1. MARKET VALUE (USD MILLION)
19.5.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.5.3.3. AVERAGE TEST COST (USD)
19.5.4 OTHERS
19.6 TISSUE TYPING TEST
19.6.1 POLYMERASE CHAIN REACTION (PCR)
19.6.1.1. MARKET VALUE (USD MILLION)
19.6.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.1.3. AVERAGE TEST COST (USD)
19.6.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.6.2.1. MARKET VALUE (USD MILLION)
19.6.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.2.3. AVERAGE TEST COST (USD)
19.6.3 NEXT GENERATION SEQUENCING (NGS)
19.6.3.1. MARKET VALUE (USD MILLION)
19.6.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.3.3. AVERAGE TEST COST (USD)
19.6.4 CYTOGENETICS
19.6.4.1. MARKET VALUE (USD MILLION)
19.6.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.4.3. AVERAGE TEST COST (USD)
19.6.5 CAPILLARY ELECTROPHORESIS
19.6.5.1. MARKET VALUE (USD MILLION)
19.6.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.5.3. AVERAGE TEST COST (USD)
19.6.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.6.6.1. MARKET VALUE (USD MILLION)
19.6.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.6.3. AVERAGE TEST COST (USD)
19.6.7 MOLECULAR IMAGING
19.6.7.1. MARKET VALUE (USD MILLION)
19.6.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.7.3. AVERAGE TEST COST (USD)
19.6.8 MASS SPECTROMETRY (MS)
19.6.8.1. MARKET VALUE (USD MILLION)
19.6.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.8.3. AVERAGE TEST COST (USD)
19.6.9 CHIPS AND MICROARRAY
19.6.9.1. MARKET VALUE (USD MILLION)
19.6.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.9.3. AVERAGE TEST COST (USD)
19.6.10 OTHERS
19.7 BLOOD SCREENING
19.7.1 POLYMERASE CHAIN REACTION (PCR)
19.7.1.1. MARKET VALUE (USD MILLION)
19.7.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.1.3. AVERAGE TEST COST (USD)
19.7.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.7.2.1. MARKET VALUE (USD MILLION)
19.7.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.2.3. AVERAGE TEST COST (USD)
19.7.3 NEXT GENERATION SEQUENCING (NGS)
19.7.3.1. MARKET VALUE (USD MILLION)
19.7.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.3.3. AVERAGE TEST COST (USD)
19.7.4 CYTOGENETICS
19.7.4.1. MARKET VALUE (USD MILLION)
19.7.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.4.3. AVERAGE TEST COST (USD)
19.7.5 CAPILLARY ELECTROPHORESIS
19.7.5.1. MARKET VALUE (USD MILLION)
19.7.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.5.3. AVERAGE TEST COST (USD)
19.7.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.7.6.1. MARKET VALUE (USD MILLION)
19.7.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.6.3. AVERAGE TEST COST (USD)
19.7.7 MOLECULAR IMAGING
19.7.7.1. MARKET VALUE (USD MILLION)
19.7.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.7.3. AVERAGE TEST COST (USD)
19.7.8 MASS SPECTROMETRY (MS)
19.7.8.1. MARKET VALUE (USD MILLION)
19.7.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.8.3. AVERAGE TEST COST (USD)
19.7.9 CHIPS AND MICROARRAY
19.7.9.1. MARKET VALUE (USD MILLION)
19.7.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.9.3. AVERAGE TEST COST (USD)
19.7.10 OTHERS
19.8 CARDIOVASCULAR DISEASES TESTING
19.8.1 POLYMERASE CHAIN REACTION (PCR)
19.8.1.1. MARKET VALUE (USD MILLION)
19.8.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.1.3. AVERAGE TEST COST (USD)
19.8.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.8.2.1. MARKET VALUE (USD MILLION)
19.8.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.2.3. AVERAGE TEST COST (USD)
19.8.3 NEXT GENERATION SEQUENCING (NGS)
19.8.3.1. MARKET VALUE (USD MILLION)
19.8.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.3.3. AVERAGE TEST COST (USD)
19.8.4 CYTOGENETICS
19.8.4.1. MARKET VALUE (USD MILLION)
19.8.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.4.3. AVERAGE TEST COST (USD)
19.8.5 CAPILLARY ELECTROPHORESIS
19.8.5.1. MARKET VALUE (USD MILLION)
19.8.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.5.3. AVERAGE TEST COST (USD)
19.8.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.8.6.1. MARKET VALUE (USD MILLION)
19.8.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.6.3. AVERAGE TEST COST (USD)
19.8.7 MOLECULAR IMAGING
19.8.7.1. MARKET VALUE (USD MILLION)
19.8.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.7.3. AVERAGE TEST COST (USD)
19.8.8 MASS SPECTROMETRY (MS)
19.8.8.1. MARKET VALUE (USD MILLION)
19.8.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.8.3. AVERAGE TEST COST (USD)
19.8.9 CHIPS AND MICROARRAY
19.8.9.1. MARKET VALUE (USD MILLION)
19.8.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.9.3. AVERAGE TEST COST (USD)
19.8.10 OTHERS
19.9 NEUROLOGICAL DISEASES TESTING
19.9.1 POLYMERASE CHAIN REACTION (PCR)
19.9.1.1. MARKET VALUE (USD MILLION)
19.9.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.1.3. AVERAGE TEST COST (USD)
19.9.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.9.2.1. MARKET VALUE (USD MILLION)
19.9.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.2.3. AVERAGE TEST COST (USD)
19.9.3 NEXT GENERATION SEQUENCING (NGS)
19.9.3.1. MARKET VALUE (USD MILLION)
19.9.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.3.3. AVERAGE TEST COST (USD)
19.9.4 CYTOGENETICS
19.9.4.1. MARKET VALUE (USD MILLION)
19.9.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.4.3. AVERAGE TEST COST (USD)
19.9.5 CAPILLARY ELECTROPHORESIS
19.9.5.1. MARKET VALUE (USD MILLION)
19.9.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.5.3. AVERAGE TEST COST (USD)
19.9.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.9.6.1. MARKET VALUE (USD MILLION)
19.9.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.6.3. AVERAGE TEST COST (USD)
19.9.7 MOLECULAR IMAGING
19.9.7.1. MARKET VALUE (USD MILLION)
19.9.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.7.3. AVERAGE TEST COST (USD)
19.9.8 MASS SPECTROMETRY (MS)
19.9.8.1. MARKET VALUE (USD MILLION)
19.9.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.8.3. AVERAGE TEST COST (USD)
19.9.9 CHIPS AND MICROARRAY
19.9.9.1. MARKET VALUE (USD MILLION)
19.9.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.9.3. AVERAGE TEST COST (USD)
19.9.10 OTHERS
19.1 INFECTIOUS DISEASES TESTING
19.10.1 BY TYPE
19.10.1.1. COVID-19
19.10.1.1.1. MARKET VALUE (USD MILLION)
19.10.1.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.1.3. AVERAGE TEST COST (USD)
19.10.1.2. HEPATITIS
19.10.1.2.1. MARKET VALUE (USD MILLION)
19.10.1.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.2.3. AVERAGE TEST COST (USD)
19.10.1.3. HIV
19.10.1.3.1. MARKET VALUE (USD MILLION)
19.10.1.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.3.3. AVERAGE TEST COST (USD)
19.10.1.4. CT/NG
19.10.1.4.1. MARKET VALUE (USD MILLION)
19.10.1.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.4.3. AVERAGE TEST COST (USD)
19.10.1.5. HAI
19.10.1.5.1. MARKET VALUE (USD MILLION)
19.10.1.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.5.3. AVERAGE TEST COST (USD)
19.10.1.6. HPV
19.10.1.6.1. MARKET VALUE (USD MILLION)
19.10.1.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.6.3. AVERAGE TEST COST (USD)
19.10.1.7. TUBERCULOSIS
19.10.1.7.1. MARKET VALUE (USD MILLION)
19.10.1.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.7.3. AVERAGE TEST COST (USD)
19.10.1.8. INFLUENZA
19.10.1.8.1. MARKET VALUE (USD MILLION)
19.10.1.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.8.3. AVERAGE TEST COST (USD)
19.10.1.9. VECTOR-BORNE DISEASE
19.10.1.9.1. MALERIA
19.10.1.9.1.1 MARKET VALUE (USD MILLION)
19.10.1.9.1.2 MARKET VOLUME (NUMBER OF TESTS)
19.10.1.9.1.3 AVERAGE TEST COST (USD)
19.10.1.9.2. DENGUE
19.10.1.9.2.1 MARKET VALUE (USD MILLION)
19.10.1.9.2.2 MARKET VOLUME (NUMBER OF TESTS)
19.10.1.9.2.3 AVERAGE TEST COST (USD)
19.10.1.9.3. ZIKA VIRUS
19.10.1.9.3.1 MARKET VALUE (USD MILLION)
19.10.1.9.3.2 MARKET VOLUME (NUMBER OF TESTS)
19.10.1.9.3.3 AVERAGE TEST COST (USD)
19.10.1.9.4. OTHERS
19.10.2 BY TECHNOLOGY
19.10.2.1. POLYMERASE CHAIN REACTION (PCR)
19.10.2.1.1. MARKET VALUE (USD MILLION)
19.10.2.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.1.3. AVERAGE TEST COST (USD)
19.10.2.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.10.2.2.1. MARKET VALUE (USD MILLION)
19.10.2.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.2.3. AVERAGE TEST COST (USD)
19.10.2.3. NEXT GENERATION SEQUENCING (NGS)
19.10.2.3.1. MARKET VALUE (USD MILLION)
19.10.2.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.3.3. AVERAGE TEST COST (USD)
19.10.2.4. CYTOGENETICS
19.10.2.4.1. MARKET VALUE (USD MILLION)
19.10.2.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.4.3. AVERAGE TEST COST (USD)
19.10.2.5. CAPILLARY ELECTROPHORESIS
19.10.2.5.1. MARKET VALUE (USD MILLION)
19.10.2.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.5.3. AVERAGE TEST COST (USD)
19.10.2.6. IN SITU HYBRIDIZATION (ISH OR FISH)
19.10.2.6.1. MARKET VALUE (USD MILLION)
19.10.2.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.6.3. AVERAGE TEST COST (USD)
19.10.2.7. MOLECULAR IMAGING
19.10.2.7.1. MARKET VALUE (USD MILLION)
19.10.2.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.7.3. AVERAGE TEST COST (USD)
19.10.2.8. MASS SPECTROMETRY (MS)
19.10.2.8.1. MARKET VALUE (USD MILLION)
19.10.2.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.8.3. AVERAGE TEST COST (USD)
19.10.2.9. CHIPS AND MICROARRAY
19.10.2.9.1. MARKET VALUE (USD MILLION)
19.10.2.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.9.3. AVERAGE TEST COST (USD)
19.10.2.10. OTHERS
19.11 OTHERS
20 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY TESTING SITE
20.1 OVERVIEW
20.2 LABORATORY BASED
20.3 POINT OF CARE TESTING
21 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS CORE LABORATORIES
21.3 HOSPITAL ALTERNATIVE SITES
21.3.1 CLINICS
21.3.2 REFERENCE LABORATORIES
21.3.3 DIAGNOSTIC CENTERS
21.3.4 ACADEMIC & RESEARCH INSTITUTES
21.3.5 OTHERS
21.4 DECENTRALIZED TEST SITES
21.4.1 DECENTRALIZED CLINICAL LABORATORIES
21.4.2 PHARMACIES
21.4.3 HOMECARE SETTING
21.5 OTHERS
22 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDERS
22.3 RETAIL SALES
22.3.1 ONLINE SALES
22.3.2 OFFLINES SALES
22.4 OTHERS
23 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY
GLOBAL MOLECULAR DIAGNOSTICS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 NORTH AMERICA
23.1.1 U.S.
23.1.2 CANADA
23.1.3 MEXICO
23.2 EUROPE
23.2.1 GERMANY
23.2.2 FRANCE
23.2.3 U.K.
23.2.4 ITALY
23.2.5 SPAIN
23.2.6 RUSSIA
23.2.7 TURKEY
23.2.8 BELGIUM
23.2.9 NETHERLANDS
23.2.10 SWITZERLAND
23.2.11 REST OF EUROPE
23.3 ASIA-PACIFIC
23.3.1 JAPAN
23.3.2 CHINA
23.3.3 SOUTH KOREA
23.3.4 INDIA
23.3.5 AUSTRALIA
23.3.6 SINGAPORE
23.3.7 THAILAND
23.3.8 MALAYSIA
23.3.9 INDONESIA
23.3.10 PHILIPPINES
23.3.11 REST OF ASIA-PACIFIC
23.4 SOUTH AMERICA
23.4.1 BRAZIL
23.4.2 ARGENTINA
23.4.3 PERU
23.4.4 CHILE
23.4.5 COLOMBIA
23.4.6 VENEZUELA
23.4.7 REST OF SOUTH AMERICA
23.5 MIDDLE EAST AND AFRICA
23.5.1 SOUTH AFRICA
23.5.2 SAUDI ARABIA
23.5.3 UAE
23.5.4 EGYPT
23.5.5 ISRAEL
23.5.6 REST OF MIDDLE EAST AND AFRICA
23.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 GLOBAL SWINE AND POULTRY RESPIRATORY DISEASES TREATMENT MARKET , COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL MOLECULAR DIAGNOSTICS MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.2 MERGERS & ACQUISITIONS
25.3 NEW PRODUCT DEVELOPMENT & APPROVALS
25.4 EXPANSIONS
25.5 REGULATORY CHANGES
25.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL MOLECULAR DIAGNOSTICS MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL MOLECULAR DIAGNOSTICS MARKET, COMPANY PROFILE
27.1 ABBOTT
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 SIEMENS HEALTHCARE PRIVATE LIMITED
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 THERMO FISHER SCIENTIFIC INC.
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 BD
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 BIOMÉRIEUX SA
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 DANAHER CORPORATION
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 HOLOGIC, INC.
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 MYRIAD GENETICS, INC.
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 QIAGEN
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 AGILENT TECHNOLOGIES, INC.
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 QUIDEL CORPORATION.
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 BIO-RAD LABORATORIES, INC.
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ILLUMINA, INC.
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 IMMUCOR (WERFEN, S.A.)
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 DIASORIN S.P.A
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 SD BIOSENSOR
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 F. HOFFMANN-LA ROCHE LTD
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 GENEPATH DIAGNOSTICS
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 EXACT SCIENCES CORPORATION
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 GUARDANT HEALTH
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 REVVITY INC.
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 CARIS LIFE SCIENCES.
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 RANDOX LABORATORIES LTD.
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 DAAN GENE CO., LTD.
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 JIANGSU MACRO & MICRO-TEST MED-TECH CO., LTD.
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 GENOBIO PHARMACEUTICAL CO., LTD. (ERA BIOLOGY GROUP)
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 LEPU MEDICAL TECHNOLOGY(BEIJING)CO.,LTD.
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 PROMEGA CORPORATION
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
27.29 TRIPLEX INTERNATIONAL BIOSCIENCES CO. LTD.
27.29.1 COMPANY OVERVIEW
27.29.2 REVENUE ANALYSIS
27.29.3 GEOGRAPHIC PRESENCE
27.29.4 PRODUCT PORTFOLIO
27.29.5 RECENT DEVELOPMENTS
27.3 MGMED
27.30.1 COMPANY OVERVIEW
27.30.2 REVENUE ANALYSIS
27.30.3 GEOGRAPHIC PRESENCE
27.30.4 PRODUCT PORTFOLIO
27.30.5 RECENT DEVELOPMENTS
27.31 MOLBIO DIAGNOSTICS PVT. LTD.
27.31.1 COMPANY OVERVIEW
27.31.2 REVENUE ANALYSIS
27.31.3 GEOGRAPHIC PRESENCE
27.31.4 PRODUCT PORTFOLIO
27.31.5 RECENT DEVELOPMENTS
27.32 GENOME DIAGNOSTICS PVT. LTD.
27.32.1 COMPANY OVERVIEW
27.32.2 REVENUE ANALYSIS
27.32.3 GEOGRAPHIC PRESENCE
27.32.4 PRODUCT PORTFOLIO
27.32.5 RECENT DEVELOPMENTS
27.33 VELA DIAGNOSTICS
27.33.1 COMPANY OVERVIEW
27.33.2 REVENUE ANALYSIS
27.33.3 GEOGRAPHIC PRESENCE
27.33.4 PRODUCT PORTFOLIO
27.33.5 RECENT DEVELOPMENTS
27.34 SHANGHAI CHUANGKUN BIO
27.34.1 COMPANY OVERVIEW
27.34.2 REVENUE ANALYSIS
27.34.3 GEOGRAPHIC PRESENCE
27.34.4 PRODUCT PORTFOLIO
27.34.5 RECENT DEVELOPMENTS
27.35 TRANSGEN BIOTECH CO., LTD.
27.35.1 COMPANY OVERVIEW
27.35.2 REVENUE ANALYSIS
27.35.3 GEOGRAPHIC PRESENCE
27.35.4 PRODUCT PORTFOLIO
27.35.5 RECENT DEVELOPMENTS
27.36 NANJING VAZYME BIOTECH CO.,LTD.
27.36.1 COMPANY OVERVIEW
27.36.2 REVENUE ANALYSIS
27.36.3 GEOGRAPHIC PRESENCE
27.36.4 PRODUCT PORTFOLIO
27.36.5 RECENT DEVELOPMENTS
27.37 WUXI NEST BIOTECHNOLOGY CO.,LTD
27.37.1 COMPANY OVERVIEW
27.37.2 REVENUE ANALYSIS
27.37.3 GEOGRAPHIC PRESENCE
27.37.4 PRODUCT PORTFOLIO
27.37.5 RECENT DEVELOPMENTS
27.38 HANGZHOU BIGFISH BIO-TECH CO., LTD.
27.38.1 COMPANY OVERVIEW
27.38.2 REVENUE ANALYSIS
27.38.3 GEOGRAPHIC PRESENCE
27.38.4 PRODUCT PORTFOLIO
27.38.5 RECENT DEVELOPMENTS
27.39 MAGGENOME TECHNOLOGIES PVT. LTD.
27.39.1 COMPANY OVERVIEW
27.39.2 REVENUE ANALYSIS
27.39.3 GEOGRAPHIC PRESENCE
27.39.4 PRODUCT PORTFOLIO
27.39.5 RECENT DEVELOPMENTS
27.4 YANENG BIOSCIENCE (SHENZHEN) CO., LTD.
27.40.1 COMPANY OVERVIEW
27.40.2 REVENUE ANALYSIS
27.40.3 GEOGRAPHIC PRESENCE
27.40.4 PRODUCT PORTFOLIO
27.40.5 RECENT DEVELOPMENTS
27.41 MERIDIAN BIOSCIENCE
27.41.1 COMPANY OVERVIEW
27.41.2 REVENUE ANALYSIS
27.41.3 GEOGRAPHIC PRESENCE
27.41.4 PRODUCT PORTFOLIO
27.41.5 RECENT DEVELOPMENTS
28 RELATED REPORTS
29 CONCLUSION
30 QUESTIONNAIRE
31 ABOUT DATA BRIDGE MARKET RESEARCH

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。