Global Pharma E Commerce Market
市场规模(十亿美元)
CAGR :
% 
 USD
14.70 Billion
USD
74.57 Billion
2024
2032
USD
14.70 Billion
USD
74.57 Billion
2024
2032
| 2025 –2032 | |
| USD 14.70 Billion | |
| USD 74.57 Billion | |
|
|
|
|
全球醫藥電子商務市場細分,按產品(處方藥和非處方藥)、最終用戶(直銷、分銷商和線上) - 行業趨勢和預測至 2032 年
醫藥電子商務市場規模
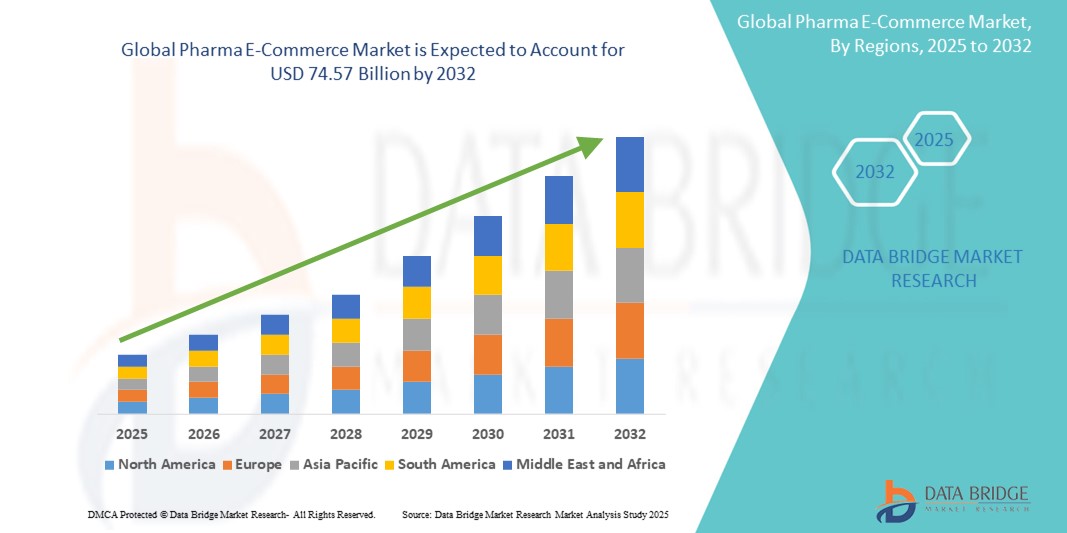
- 2024 年全球醫藥電子商務市場價值為147 億美元,預計到 2032 年將達到 745.7 億美元
- 在 2025 年至 2032 年的預測期內,市場可能會以22.50% 的複合年增長率成長,這主要得益於全球醫藥電子商務市場越來越多地採用線上醫藥平台
- 這一增長受到消費者對網上購物的偏好日益增長、新興經濟體互聯網普及率不斷提高以及藥品送貨上門需求不斷增長等因素的推動。
醫藥電子商務市場分析
- 消費者越來越傾向於網路藥局,因為他們尋求送貨上門的便利性以及輕鬆比較價格和產品的能力
- 電子商務平台正在整合人工智慧和數據分析等技術,以提供個人化推薦並改善客戶體驗
- 儘管市場正在成長,但仍面臨與假藥和監管合規相關的挑戰,需要採取嚴格措施確保消費者安全
- 例如,亞馬遜進軍醫藥電子商務領域,提供處方藥和保健產品,從而擴大其醫療保健產品組合
- 醫藥電子商務市場正在快速發展,技術進步和消費者偏好的轉變在其擴張中發揮關鍵作用
報告範圍和醫藥電子商務市場細分
|
屬性 |
醫藥電子商務關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
北美洲
歐洲
亞太
中東和非洲
南美洲
|
|
主要市場參與者 |
|
|
市場機會 |
|
|
加值資料資訊集 |
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深入的專家分析、患者流行病學、管道分析、定價分析和監管框架。 |
醫藥電子商務市場趨勢
“遠距醫療與醫藥電商的融合”
- 醫藥電商平台越來越多地整合遠距醫療服務,以提供全面的醫療保健體驗。這種整合使患者能夠遠端諮詢醫療保健提供者,並收到可直接由線上藥局配藥的處方。
- 例如,輝瑞計劃推出一個線上直銷平台,將美國客戶與獨立的遠距醫療顧問和藥品配藥合作夥伴聯繫起來,旨在簡化 Paxlovid 和 Zazpret 等藥物的取得。
- 消費者正在尋求更便利的醫療保健方式,從而推動了將遠距醫療與醫藥服務結合的平台的需求
- 例如,亞馬遜藥局正在經歷快速增長,部分原因是對抗肥胖藥物的需求量很大,並且正在探索當日送達和潛在的無人機送貨等進步,以提高客戶的便利性
- 人工智慧和數據分析等技術的進步,使電商平台能夠提供個人化的醫療保健服務。這些技術能夠根據個人健康狀況和先前購買記錄開藥,從而改善患者的整體體驗。
- 藥廠與電商平台的策略合作正在促進遠距醫療服務的整合。這些合作旨在將醫療諮詢與便利的藥物取得相結合,提供無縫的醫療體驗,從而擴大醫療服務的覆蓋範圍和有效性。
醫藥電子商務市場動態
司機
“網上藥局的普及率不斷提高”
- 全球電子商務趨勢對醫藥產業產生了重大影響,越來越多的消費者選擇網路藥局,因為網路藥局送貨上門很方便,而且可以輕鬆比較價格
- 醫藥電商平台越來越多地整合遠距醫療服務,使患者能夠遠距諮詢醫療保健提供者,並獲得可直接由線上藥局配藥的處方。
- 人工智慧和數據分析的應用使網路藥局能夠提供個人化推薦,改善客戶服務,提升整體用戶體驗。
- 各地監管改革正在簡化網路處方流程,確保對數位藥局進行更好的監管,為醫藥電子商務的發展創造有利環境
- 智慧型手機和網路在新興市場的日益普及為網路藥局擴大覆蓋範圍和客戶群提供了巨大的成長機會
機會
“新興市場的擴張”
- 新興市場互聯網普及率的不斷提高,為網路藥局提供了龐大的客戶群,促進了市場擴張。
- 新興市場的政府正在採取措施推廣數位健康解決方案,為醫藥電子商務的發展創造有利環境。
- 線上藥局與本地醫療保健提供者之間的合作可以增強服務交付,並擴大新興市場不同地區的覆蓋範圍。
- 客製化服務以滿足新興市場消費者的特定需求和偏好,可以提高客戶的採用率和忠誠度
- 網路藥局可以提供經濟高效的藥品取得解決方案,解決新興市場普遍存在的藥品負擔能力問題
克制/挑戰
“假藥”
- 對於尋求全球擴張的線上藥局來說,應對不同地區複雜多樣的監管要求是一項重大挑戰。
- 醫藥電商市場假藥氾濫,引發病人安全擔憂,損害消費者信任
- 確保敏感患者資訊的安全並遵守資料保護法規是網路藥局面臨的關鍵挑戰
- 實施嚴格的驗證流程來確認網路藥局的合法性可能會減緩市場擴張並增加營運成本
- 對藥品真實性和網路藥局可靠性的擔憂可能會阻止消費者採用電子藥局服務,從而限制市場成長
醫藥電子商務市場範圍
市場根據產品和最終用戶進行細分。
|
分割 |
細分 |
|
按產品 |
|
|
按最終用戶 |
|
醫藥電子商務市場區域分析
“北美是醫藥電子商務市場的主導地區”
- 美國是主要推動力,越來越多的美國人轉向網路藥局購買處方藥和非處方藥
- 該地區受益於先進的醫療基礎設施、高網路普及率和優惠的監管政策
- 嚴格的資料隱私法規(例如 HIPAA)的實施增強了人們對電子商務平台的信任,確保了消費者對在線購買藥品的信心
- 對健康產品、營養保健品和醫療保健服務便利性的高需求支撐了該地區的持續成長
“亞太地區預計將實現最高成長率”
- 中國、印度和日本等國家處於這一擴張的前沿,得益於強大的基礎設施和政府推動數位健康的舉措
- 該地區人口眾多,加上對藥品、保健產品和線上醫療服務的需求不斷增長,帶來了巨大的成長機會
- 新興市場實體藥局有限,為電商平台提供了尚未開發的潛力
- 該地區的監管機構正在逐步改善對網路藥品銷售的立場,為電子商務公司創造更安全、更有條理的環境
醫藥電子商務市場份額
市場競爭格局按競爭對手提供詳細資料。詳細資訊包括公司概況、公司財務狀況、收入、市場潛力、研發投入、新市場計劃、全球影響力、生產基地和設施、生產能力、公司優勢和劣勢、產品發布、產品寬度和廣度以及應用主導地位。以上提供的數據點僅與公司在市場中的重點相關。
市場中主要的市場領導者有:
- 勃林格殷格翰國際有限公司(德國)
- 艾伯維公司(美國)
- 奧羅賓多製藥(印度)
- 輝瑞公司(美國)
- 沃爾瑪公司(美國)
- 沃爾格林公司(美國)
- 快速藥方(美國)
- 克羅格公司(美國)
- L Rowland & Co(英國)
- DocMorris(瑞士)
- Giant Eagle, Inc.(美國)
- OptumRx, Inc.(美國)
- CVS Health(美國)
- 默克公司(美國)
- 賽默飛世爾科技(美國)
全球醫藥電子商務市場的最新發展
- 2024 年 10 月,亞馬遜 藥局在洛杉磯和紐約市推出了當日送達處方服務,並計劃在 2024 年底擴展到另外 12 個城市。這項服務利用人工智慧和機器學習來加快處方配送速度,提高客戶便利性
- 2024 年 3 月,禮 來公司與亞馬遜 藥局合作,透過其直接面向消費者的網站配送減肥藥 Zepbound 和其他藥品
- 2024 年 1 月,禮 來公司在美國為肥胖、偏頭痛和糖尿病患者推出了LillyDirect。該平台為美國民眾提供疾病管理資源,並將部分禮來藥品直接送到家中
- 2024年12月,加拿大金融科技公司Nuvei Corporation與Familiprix合作,推出了一項電子商務計劃,允許合作藥局接受線上支付。該計劃旨在提高支付接受率,並簡化線上購物者的結帳流程,使魁北克省和新不倫瑞克省的400多家獨立藥局能夠進入線上市場。
- 2023年3月,羅曼 製藥公司宣布計劃推出一家直接面向消費者 (D2C) 的線上商店,以此作為銷售成長的途徑。該平台提供各種營養保健產品,例如維生素、礦物質、草藥補充劑和其他營養補充劑,為消費者提供優質產品的實用途徑。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL PHARMA E-COMMERCE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL PHARMA E-COMMERCE MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL PHARMA E-COMMERCE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER'S FIVE FORCES MODEL
5.3 E-COMMERCE STRATEGIES BY PHARMACEUTICAL MANUFACTURERS
5.3.1 US
5.3.1.1. GSK
5.3.1.2. NOVARTIS
5.3.1.3. MERCK
5.3.1.4. PFIZER
5.3.1.5. ASTRAZENECA
5.3.1.6. BMS
5.3.1.7. ABBVIE
5.3.1.8. AMGEN
5.3.1.9. ROCHE
5.3.1.10. NOVONORDISK
5.3.1.11. LILLY
5.3.1.12. VIATRIS
5.3.1.13. ORGANON
5.3.1.14. OTHERS
5.3.2 REST OF THE WORLD
5.3.2.1. GSK
5.3.2.2. NOVARTIS
5.3.2.3. MERCK
5.3.2.4. PFIZER
5.3.2.5. ASTRAZENECA
5.3.2.6. BMS
5.3.2.7. ABBVIE
5.3.2.8. AMGEN
5.3.2.9. ROCHE
5.3.2.10. NOVONORDISK
5.3.2.11. LILLY
5.3.2.12. VIATRIS
5.3.2.13. ORGANON
5.3.2.14. OTHERS
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYSIS AND RECOMMENDATIONS
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNOLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 GLOBAL PHARMA E-COMMERCE MARKET, BY PRODUCT
17.1 OVERVIEW
17.2 PRESCRIPTION PHARMACEUTICALS
17.2.1 ANALGESICS
17.2.1.1. PARACETAMOL
17.2.1.2. FENTANYL
17.2.1.3. TRAMADOL
17.2.1.4. ACETAMINOPHEN
17.2.1.5. OTHERS
17.2.2 IMMUNOSUPPRESSIVES
17.2.2.1. AZATHIOPRINE
17.2.2.2. CICLOSPORIN
17.2.2.3. MYCOPHENOLATE MOFETIL
17.2.2.4. OTHERS
17.2.3 ANTIDEPRESSANT
17.2.3.1. RISPERIDONE
17.2.3.2. OLANZAPINE
17.2.3.3. TRIFLUOPERAZINE
17.2.3.4. CHLORDIAZEPOXIDE
17.2.3.5. OTHERS
17.2.4 ANTI-INFECTIVES
17.2.4.1. ANTIBIOTICS
17.2.4.1.1. AMOXICILLIN
17.2.4.1.2. METRONIDAZOLE
17.2.4.1.3. DOXYCYCLINE
17.2.4.1.4. CIPROFLOXACIN
17.2.4.1.5. LEVOFLOXACIN
17.2.4.1.6. OTHERS
17.2.4.2. ANTIVIRAL
17.2.4.2.1. ACICLOVIR
17.2.4.2.2. ABACAVIR
17.2.4.2.3. LOPINAVIR
17.2.4.2.4. OSELTAMIVIR
17.2.4.2.5. OTHERS
17.2.4.3. ANTIFUNGAL
17.2.4.3.1. CLOTRIMAZOLE
17.2.4.3.2. ITRACONAZOLE
17.2.4.3.3. NYSTATIN
17.2.4.3.4. VORICONAZOLE
17.2.4.3.5. FLUCONAZOLE
17.2.4.3.6. OTHERS
17.2.4.4. OTHERS
17.2.5 ANTI-INFLAMMATORY
17.2.5.1. DICLOFENAC
17.2.5.2. IBUPROFEN
17.2.5.3. NAPROXEN
17.2.5.4. PREDNISOLONE
17.2.5.5. OTHERS
17.2.6 DIURETICS
17.2.6.1. SPIRONOLACTONE
17.2.6.2. HYDROCHLOROTHIAZIDE
17.2.6.3. FUROSEMIDE
17.2.6.4. OTHERS
17.2.7 GASTROINTESTINAL
17.2.7.1. ESOMOPRAZOLE
17.2.7.2. OMEPRAZOLE
17.2.7.3. RANITIDINE
17.2.7.4. LOPERAMIDE
17.2.7.5. OTHERS
17.2.8 CARDIOVASCULAR
17.2.8.1. ATENOLOL
17.2.8.2. MOLSIDOMINE
17.2.8.3. DIGOXIN
17.2.8.4. VERAPAMIL
17.2.8.5. OTHERS
17.3 VACCINES
17.3.1 EBOLA VACCINE
17.3.2 HUMAN PAPILLOMAVIRUS VACCINE
17.3.3 MEASLES, MUMPS, AND RUBELLA VIRUS VACCINE
17.3.4 HAEMOPHILUS B VACCINE
17.3.5 PNEUMOCOCCAL VACCINE
17.3.6 HEPATITIS B VACCINE
17.3.7 ROTAVIRUS VACCINE
17.3.8 HEPATITIS A VACCINE
17.3.9 BCG VACCINE
17.3.10 VARICELLA VIRUS VACCINE
17.3.11 DIPHTHERIA AND TETANUS VACCINES
17.3.12 SHINGLES VACCINES
17.3.13 OTHERS
17.4 SPECIALTY PHARMACEUTICALS
17.4.1 GASTROINTESTINAL
17.4.1.1. AFINITOR
17.4.1.2. AYVAKIT
17.4.1.3. BRAFTOVI
17.4.1.4. CYRAMZA
17.4.1.5. ENHERTU
17.4.1.6. LEUCOVORIN CALCIUM
17.4.1.7. LENVIMA
17.4.1.8. LONSURF
17.4.1.9. LYNPARZA
17.4.1.10. NEXAVAR
17.4.1.11. PEMAZYRE
17.4.1.12. QINLOCK
17.4.1.13. STIVARGA
17.4.1.14. SUTENT
17.4.1.15. TARCEVA
17.4.1.16. TECENTRIQ
17.4.1.17. TIBSOVO
17.4.1.18. WELIREG
17.4.1.19. XELODA
17.4.1.20. XERMELO
17.4.2 BRAIN
17.4.2.1. GLEOSTINE
17.4.2.2. MATULANE
17.4.2.3. TEMODAR
17.4.3 BREAST
17.4.3.1. AFINITOR
17.4.3.2. ANASTROZOLE
17.4.3.3. AROMASIN
17.4.3.4. ENHERTU
17.4.3.5. EVEROLIMUS
17.4.3.6. FEMARA
17.4.3.7. IBRANCE
17.4.3.8. KISQALI
17.4.3.9. LYNPARZA
17.4.3.10. MEGACE
17.4.3.11. METHOTREXATE
17.4.3.12. NERLYNX
17.4.3.13. ORSERDU
17.4.3.14. PIQRAY
17.4.3.15. SOLTAMOX
17.4.3.16. TALZENNA
17.4.3.17. TRODELVY
17.4.3.18. TUKYSA
17.4.3.19. TYKERB
17.4.3.20. VERZENIO
17.4.3.21. XELODA
17.4.3.22. OTHERS
17.4.4 GENITOURINARY
17.4.4.1. AFINITOR
17.4.4.2. BAVENCIO
17.4.4.3. CABOMETYX
17.4.4.4. CASODEX
17.4.4.5. ERLEADA
17.4.4.6. FOTIVDA
17.4.4.7. INLYTA
17.4.4.8. LENVIMA
17.4.4.9. LUPRON
17.4.4.10. LYNPARZA
17.4.4.11. NEXAVAR
17.4.4.12. NILANDRON
17.4.4.13. NUBEQA
17.4.4.14. ORGOVYX
17.4.4.15. RAPAMUNE
17.4.4.16. RUBRACA
17.4.4.17. SUTENT
17.4.4.18. TRODELVY
17.4.4.19. VOTRIENT
17.4.4.20. WELIREG
17.4.4.21. XTANDI
17.4.4.22. YONSA
17.4.4.23. ZYTIGA
17.4.5 GYNECOLOGY
17.4.5.1. HEXALEN
17.4.5.2. HYDREA
17.4.5.3. ELAHERE
17.4.5.4. LENVIMA
17.4.5.5. LYNPARZA
17.4.5.6. RUBRACA
17.4.5.7. ZEJULA
17.4.6 HEAD, NECK & THYROID
17.4.6.1. CAPRELSA
17.4.6.2. GAVRETO
17.4.6.3. HYDREA
17.4.6.4. LENVIMA
17.4.6.5. NEXAVAR
17.4.6.6. RETEVMO
17.4.7 HEMATOLOGIC
17.4.7.1. AGRYLIN
17.4.7.2. ALKERAN
17.4.7.3. BESPONSA
17.4.7.4. BESREMI
17.4.7.5. BOSULIF
17.4.7.6. BRUKINSA
17.4.7.7. CALQUENCE
17.4.7.8. COPIKTRA
17.4.7.9. CYTOXAN
17.4.7.10. DOPTELET
17.4.7.11. GLEEVEC
17.4.7.12. HYDREA
17.4.7.13. IDHIFA
17.4.7.14. IMBRUVICA
17.4.7.15. INQOVI
17.4.7.16. INREBIC
17.4.7.17. JAKAFI
17.4.7.18. JAYPIRCA
17.4.7.19. LEUCOVORIN CALCIUM
17.4.7.20. LEUKERAN
17.4.7.21. METHOTREXATE
17.4.7.22. MONJUVI
17.4.7.23. MYLOTARG
17.4.7.24. NINLARO
17.4.7.25. ONUREG
17.4.7.26. POLIVY
17.4.7.27. POMALYST
17.4.7.28. PROMACTA
17.4.7.29. PURINETHOL
17.4.7.30. REVLIMID
17.4.7.31. REZLIDHIA
17.4.7.32. REZUROCK
17.4.7.33. RYDAPT
17.4.7.34. SARCLISA
17.4.7.35. SCEMBLIX
17.4.7.36. SPRYCEL
17.4.7.37. TARGRETIN
17.4.7.38. TASIGNA
17.4.7.39. TAVALISSE
17.4.7.40. THALOMID
17.4.7.41. TIBSOVO
17.4.7.42. TRETINOIN
17.4.7.43. VENCLEXTA
17.4.7.44. VIDAZA
17.4.7.45. VONJO
17.4.7.46. XOSPATA
17.4.7.47. XPOVIO
17.4.7.48. ZYDELIG
17.4.8 LUNG
17.4.8.1. AFINITOR
17.4.8.2. ALECENSA
17.4.8.3. ALUNBRIG
17.4.8.4. COTELLIC
17.4.8.5. CYRAMZA
17.4.8.6. ERLOTINIB
17.4.8.7. EXKIVITY
17.4.8.8. GAVRETO
17.4.8.9. HYCAMTIN
17.4.8.10. IMFINZI
17.4.8.11. IRESSA
17.4.8.12. KRAZATI
17.4.8.13. LORBRENA
17.4.8.14. LUMAKRAS
17.4.8.15. METHOTREXATE
17.4.8.16. PORTRAZZA
17.4.8.17. RETEVMO
17.4.8.18. ROZLYTREK
17.4.8.19. TABRECTA
17.4.8.20. TAGRISSO
17.4.8.21. TEPMETKO
17.4.8.22. VEPESID
17.4.8.23. VIZIMPRO
17.4.8.24. XALKORI
17.4.9 MELANOMA AND BASAL CELL
17.4.9.1. BAVENCIO
17.4.9.2. BRAFTOVI
17.4.9.3. COTELLIC
17.4.9.4. ERIVEDGE
17.4.9.5. MEKINIST
17.4.9.6. MEKTOVI
17.4.9.7. ODOMZO
17.4.9.8. TAFINLAR
17.4.9.9. ZELBORAF
17.4.10 RARE AND COMPLEX THERAPIES
17.4.10.1. CABLIVI
17.4.10.2. ENSPRYNG
17.4.10.3. FYARRO
17.4.10.4. GAMIFANT
17.4.10.5. KINERET
17.4.10.6. LIVTENCITY
17.4.10.7. LUPKYNIS
17.4.10.8. NULIBRY
17.4.10.9. PYRUKYND
17.4.10.10. SKYCLARYS
17.4.10.11. TARPEYO
17.4.10.12. TURALIO
17.4.10.13. VIJOICE
17.4.11 OTHER SPECIALTY PRODUCTS
17.5 OVER THE COUNTER PHARMACEUTICALS
17.5.1 ANTACIDS
17.5.1.1. ALUMINUM CARBONATE ANTACIDS
17.5.1.2. CALCIUM CARBONATE ANTACIDS
17.5.1.3. MAGNESIUM OXIDE ANTACIDS
17.5.1.4. SODIUM BICARBONATE ANTACIDS
17.5.1.5. SODIUM CITRATE ANTACIDS
17.5.1.6. ALUMINUM HYDROXIDE ANTACIDS
17.5.1.7. MAGNESIUM HYDROXIDE ANTACIDS
17.5.1.8. OTHERS
17.5.2 PAIN RELIEF
17.5.2.1. ASPIRIN
17.5.2.2. NAPROXEN
17.5.2.3. IBUPROFEN
17.5.2.4. ACETAMINOPHEN
17.5.2.5. OTHERS
17.5.3 VITAMINS
17.5.3.1. BIOTIN
17.5.3.2. FOLIC ACID
17.5.3.3. THIAMIN
17.5.3.4. RIBOFLAVIN
17.5.3.5. PANTOTHENIC ACID
17.5.3.6. ASCORBIC ACID
17.5.3.7. COBALAMIN
17.5.3.8. PYRIDOXINE
17.5.3.9. NIACIN
17.5.3.10. RETINOIDS AND CAROTENE
17.5.3.11. CHOLINE
17.5.3.12. CALCIFEROL
17.5.3.13. ALPHA-TOCOPHEROL
17.5.3.14. PHYLLOQUINONE
17.5.3.15. MENADIONE
17.5.3.16. OTHERS
17.5.4 MINERALS
17.5.4.1. CALCIUM
17.5.4.2. CHLORIDE
17.5.4.3. CHROMIUM
17.5.4.4. COPPER
17.5.4.5. FLUORIDE
17.5.4.6. IODINE
17.5.4.7. IRON
17.5.4.8. MAGNESIUM
17.5.4.9. MANGANESE
17.5.4.10. MOLYBDENUM
17.5.4.11. PHOSPHORUS
17.5.4.12. POTASSIUM
17.5.4.13. SELENIUM
17.5.4.14. SODIUM
17.5.4.15. SULFUR
17.5.4.16. ZINC
17.5.4.17. OTHERS
17.5.5 DIETARY SUPPLEMENTATION
17.5.5.1. FISH OIL
17.5.5.2. HYDROCIL
17.5.5.3. REGULOID
17.5.5.4. PSYLLIUM
17.5.5.5. METAMUCIL
17.5.5.6. OTHERS
18 GLOBAL PHARMA E-COMMERCE MARKET, BY DRUG TYPE
18.1 OVERVIEW
18.2 BRANDED
18.3 GENERIC
19 GLOBAL PHARMA E-COMMERCE MARKET, BY THERAPEUTICS APPLICATION
19.1 OVERVIEW
19.2 ISCHEMIC HEART DISEASE
19.2.1 PRESCRIPTION MEDICINE
19.2.2 OVER THE COUNTER
19.3 CHRONIC OBSTRUCTIVE PULMONARY DISEASE
19.3.1 PRESCRIPTION MEDICINE
19.3.2 OVER THE COUNTER
19.4 DIARRHEAL DISEASES
19.4.1 PRESCRIPTION MEDICINE
19.4.2 OVER THE COUNTER
19.5 LOWER RESPIRATORY INFECTION
19.5.1 PRESCRIPTION MEDICINE
19.5.2 OVER THE COUNTER
19.6 CEREBROVASCULAR DISEASES
19.6.1 PRESCRIPTION MEDICINE
19.6.2 OVER THE COUNTER
19.7 IRON-DEFICIENCY ANAEMIA
19.7.1 PRESCRIPTION MEDICINE
19.7.2 OVER THE COUNTER
19.8 NEONATAL PRETERM BIRTH
19.8.1 PRESCRIPTION MEDICINE
19.8.2 OVER THE COUNTER
19.9 TUBERCULOSIS
19.9.1 PRESCRIPTION MEDICINE
19.9.2 OVER THE COUNTER
19.1 SENSE ORGAN DISEASES
19.10.1 PRESCRIPTION MEDICINE
19.10.2 OVER THE COUNTER
19.11 CANCER
19.11.1 PRESCRIPTION MEDICINE
19.11.2 OVER THE COUNTER
19.12 INJURIES
19.12.1 PRESCRIPTION MEDICINE
19.12.2 OVER THE COUNTER
19.13 OPHTHALMOLOGY
19.13.1 PRESCRIPTION MEDICINE
19.13.2 OVER THE COUNTER
19.14 DERMATOLOGY
19.14.1 PRESCRIPTION MEDICINE
19.14.2 OVER THE COUNTER
19.15 NEUROLOGY
19.15.1 PRESCRIPTION MEDICINE
19.15.2 OVER THE COUNTER
19.16 ENDOCRINOLOGY
19.16.1 PRESCRIPTION MEDICINE
19.16.2 OVER THE COUNTER
19.17 GASTROINTESTINAL DISORDERS
19.17.1 PRESCRIPTION MEDICINE
19.17.2 OVER THE COUNTER
19.18 AUTOIMMUNE
19.18.1 PRESCRIPTION MEDICINE
19.18.2 OVER THE COUNTER
19.19 OTHERS
20 GLOBAL PHARMA E-COMMERCE MARKET, BY ROUTE OF ADMINISTRATION
20.1 OVERVIEW
20.2 ORAL
20.2.1 TABLETS
20.2.2 CAPSULES
20.2.3 POWDER
20.2.4 PILLS
20.2.5 SYRUPS
20.2.6 OTHERS
20.3 TOPICAL
20.3.1 SOLUTIONS
20.3.2 CREAM
20.3.3 OINTMENT
20.3.4 GELS
20.3.5 LOTIONS
20.3.6 POWDERS
20.3.7 OTHERS
20.4 PARENTERAL
20.5 INTRANASAL
20.5.1 DROPS
20.5.2 SPRAYS
20.5.3 POWDERS
20.5.4 GELS
20.5.5 OTHERS
20.6 OCULAR
20.6.1 EYE DROPS
20.6.2 SPRAYS
20.6.3 OINTMENTS
20.6.4 OTHERS
20.7 OTHERS
21 GLOBAL PHARMA E-COMMERCE MARKET, BY MODEL TYPE
21.1 OVERVIEW
21.2 B2B
21.2.1 B2B2B
21.2.2 B2B2C
21.3 B2C
22 GLOBAL PHARMA E-COMMERCE MARKET, BY PLATFORM TYPE
22.1 OVERVIEW
22.2 MANUFACTURER OWNED DIGITAL COMMERCE
22.3 DISTRIBUTOR/WHOLESALER OWNED DIGITAL COMMERCE
22.4 MANUFACTURER OWNED MARKET PLACE
22.5 3RD PARTY MARKETPLACE
22.6 RETAIL
23 GLOBAL PHARMA E-COMMERCE MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.2 COMPANY SHARE ANALYSIS: EUROPE
23.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.4 MERGERS & ACQUISITIONS
23.5 NEW PRODUCT DEVELOPMENT & APPROVALS
23.6 EXPANSIONS
23.7 REGULATORY CHANGES
23.8 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL PHARMA E-COMMERCE MARKET, SWOT AND DBMR ANALYSIS
25 GLOBAL PHARMA E-COMMERCE MARKET, BY REGION
GLOBAL PHARMA E-COMMERCE MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25.1 NORTH AMERICA
25.1.1 U.S.
25.1.2 CANADA
25.1.3 MEXICO
25.2 EUROPE
25.2.1 GERMANY
25.2.2 FRANCE
25.2.3 U.K.
25.2.4 HUNGARY
25.2.5 LITHUANIA
25.2.6 AUSTRIA
25.2.7 IRELAND
25.2.8 NORWAY
25.2.9 POLAND
25.2.10 ITALY
25.2.11 SPAIN
25.2.12 RUSSIA
25.2.13 TURKEY
25.2.14 NETHERLANDS
25.2.15 SWITZERLAND
25.2.16 REST OF EUROPE
25.3 ASIA-PACIFIC
25.3.1 JAPAN
25.3.2 CHINA
25.3.3 SOUTH KOREA
25.3.4 INDIA
25.3.5 AUSTRALIA
25.3.6 SINGAPORE
25.3.7 THAILAND
25.3.8 MALAYSIA
25.3.9 INDONESIA
25.3.10 PHILIPPINES
25.3.11 VIETNAM
25.3.12 REST OF ASIA-PACIFIC
25.4 SOUTH AMERICA
25.4.1 BRAZIL
25.4.2 ARGENTINA
25.4.3 PERU
25.4.4 COLOMBIA
25.4.5 VENEZUELA
25.4.6 REST OF SOUTH AMERICA
25.5 MIDDLE EAST AND AFRICA
25.5.1 SOUTH AFRICA
25.5.2 SAUDI ARABIA
25.5.3 UAE
25.5.4 EGYPT
25.5.5 KUWAIT
25.5.6 ISRAEL
25.5.7 REST OF MIDDLE EAST AND AFRICA
25.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
26 GLOBAL PHARMA E-COMMERCE MARKET, COMPANY PROFILE
26.1 CVS HEALTH
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 PRODUCT PORTFOLIO
26.1.5 RECENT DEVELOPMENTS
26.2 ZUR ROSE GROUP AG
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPMENTS
26.3 WALGREENS BOOTS ALLIANCE
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 PRODUCT PORTFOLIO
26.3.5 RECENT DEVELOPMENTS
26.4 TRUECOMMERCE INC.
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPMENTS
26.5 PHARMAHOPERS (WEBHOPERS INFOTECH PVT. LTD)
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPMENTS
26.6 CONSULTATION REMEDIES
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPMENTS
26.7 RETAILIO
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPMENTS
26.8 PHARMAFLAIR
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPMENTS
26.9 LINIMENT PHARMA PRIVATE LIMITED
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPMENTS
26.1 ALIBABA GROUP
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPMENTS
26.11 MERCK & CO., INC.
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPMENTS
26.12 AMAZON.COM, INC.
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPMENTS
26.13 APOTEK HJÄRTAT AB. (ICA GRUPPEN AB)
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPMENTS
26.14 THE KROGER CO.
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPMENTS
26.15 GIANT EAGLE, INC.
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPMENTS
26.16 OPTUM, INC. (UNITEDHEALTH GROUP.)
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPMENTS
26.17 REDCARE PHARMACY.
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPMENTS
26.18 WALMART.
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPMENTS
26.19 DOCMORRIS
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPMENTS
26.2 TABLETKI.UA
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPMENTS
26.21 APOLLO PHARMACY (APOLLO HOSPITALS)
26.21.1 COMPANY OVERVIEW
26.21.2 REVENUE ANALYSIS
26.21.3 GEOGRAPHIC PRESENCE
26.21.4 PRODUCT PORTFOLIO
26.21.5 RECENT DEVELOPMENTS
26.22 PHARMEASY
26.22.1 COMPANY OVERVIEW
26.22.2 REVENUE ANALYSIS
26.22.3 GEOGRAPHIC PRESENCE
26.22.4 PRODUCT PORTFOLIO
26.22.5 RECENT DEVELOPMENTS
26.23 RITE AID CORP.
26.23.1 COMPANY OVERVIEW
26.23.2 REVENUE ANALYSIS
26.23.3 GEOGRAPHIC PRESENCE
26.23.4 PRODUCT PORTFOLIO
26.23.5 RECENT DEVELOPMENTS
26.24 ASNA.
26.24.1 COMPANY OVERVIEW
26.24.2 REVENUE ANALYSIS
26.24.3 GEOGRAPHIC PRESENCE
26.24.4 PRODUCT PORTFOLIO
26.24.5 RECENT DEVELOPMENTS
26.25 MEDPLUSMART.COM.
26.25.1 COMPANY OVERVIEW
26.25.2 REVENUE ANALYSIS
26.25.3 GEOGRAPHIC PRESENCE
26.25.4 PRODUCT PORTFOLIO
26.25.5 RECENT DEVELOPMENTS
26.26 FOOD LION LLC
26.26.1 COMPANY OVERVIEW
26.26.2 REVENUE ANALYSIS
26.26.3 GEOGRAPHIC PRESENCE
26.26.4 PRODUCT PORTFOLIO
26.26.5 RECENT DEVELOPMENTS
26.27 RXSENSE (SINGLECARE)
26.27.1 COMPANY OVERVIEW
26.27.2 REVENUE ANALYSIS
26.27.3 GEOGRAPHIC PRESENCE
26.27.4 PRODUCT PORTFOLIO
26.27.5 RECENT DEVELOPMENTS
26.28 LLC FAMILY PHARMACY APRIL
26.28.1 COMPANY OVERVIEW
26.28.2 REVENUE ANALYSIS
26.28.3 GEOGRAPHIC PRESENCE
26.28.4 PRODUCT PORTFOLIO
26.28.5 RECENT DEVELOPMENTS
26.29 MCKESSON CORPORATION
26.29.1 COMPANY OVERVIEW
26.29.2 REVENUE ANALYSIS
26.29.3 GEOGRAPHIC PRESENCE
26.29.4 PRODUCT PORTFOLIO
26.29.5 RECENT DEVELOPMENTS
26.3 UK MEDS DIRECT LTD
26.30.1 COMPANY OVERVIEW
26.30.2 REVENUE ANALYSIS
26.30.3 GEOGRAPHIC PRESENCE
26.30.4 PRODUCT PORTFOLIO
26.30.5 RECENT DEVELOPMENTS
26.31 DIRK ROSSMANN GMBH
26.31.1 COMPANY OVERVIEW
26.31.2 REVENUE ANALYSIS
26.31.3 GEOGRAPHIC PRESENCE
26.31.4 PRODUCT PORTFOLIO
26.31.5 RECENT DEVELOPMENTS
26.32 AMERISOURCEBERGEN CORPORATION.
26.32.1 COMPANY OVERVIEW
26.32.2 REVENUE ANALYSIS
26.32.3 GEOGRAPHIC PRESENCE
26.32.4 PRODUCT PORTFOLIO
26.32.5 RECENT DEVELOPMENTS
26.33 STOLICHKI
26.33.1 COMPANY OVERVIEW
26.33.2 REVENUE ANALYSIS
26.33.3 GEOGRAPHIC PRESENCE
26.33.4 PRODUCT PORTFOLIO
26.33.5 RECENT DEVELOPMENTS
26.34 RAIA DROGA
26.34.1 COMPANY OVERVIEW
26.34.2 REVENUE ANALYSIS
26.34.3 GEOGRAPHIC PRESENCE
26.34.4 PRODUCT PORTFOLIO
26.34.5 RECENT DEVELOPMENTS
26.35 APTEKA.RU
26.35.1 COMPANY OVERVIEW
26.35.2 REVENUE ANALYSIS
26.35.3 GEOGRAPHIC PRESENCE
26.35.4 PRODUCT PORTFOLIO
26.35.5 RECENT DEVELOPMENTS
26.36 GOODRX
26.36.1 COMPANY OVERVIEW
26.36.2 REVENUE ANALYSIS
26.36.3 GEOGRAPHIC PRESENCE
26.36.4 PRODUCT PORTFOLIO
26.36.5 RECENT DEVELOPMENTS
26.37 CARDINAL HEALTH
26.37.1 COMPANY OVERVIEW
26.37.2 REVENUE ANALYSIS
26.37.3 GEOGRAPHIC PRESENCE
26.37.4 PRODUCT PORTFOLIO
26.37.5 RECENT DEVELOPMENTS
26.37.6
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。