North America Alpha And Beta Emitters Based Radiopharmaceuticals Market
市场规模(十亿美元)
CAGR :
% 
 USD
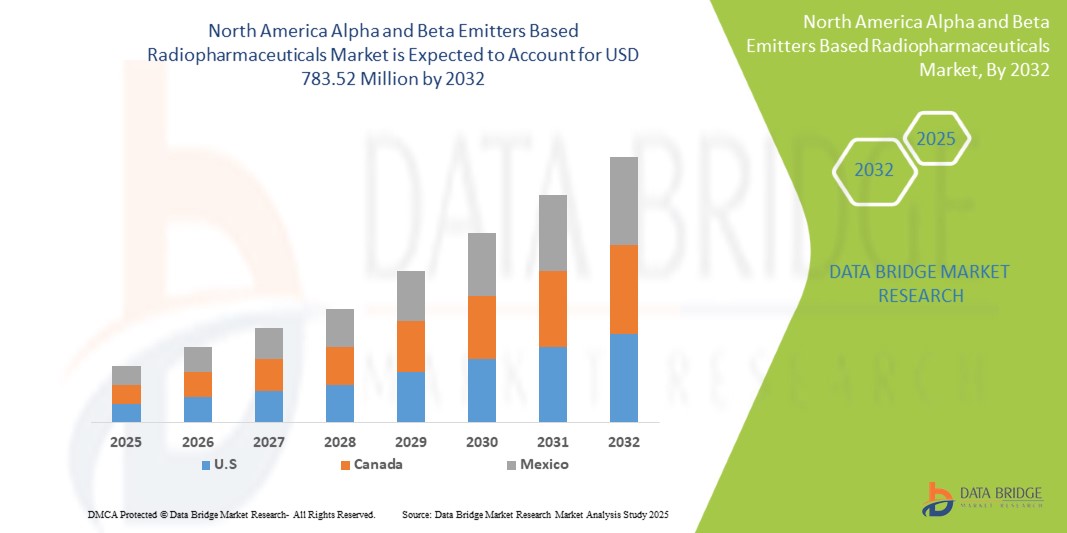
339.33 Million
USD
783.52 Million
2024
2032
USD
339.33 Million
USD
783.52 Million
2024
2032
| 2025 –2032 | |
| USD 339.33 Million | |
| USD 783.52 Million | |
|
|
|
|
北美基於 α 和 β 發射體的放射性藥物市場細分,按同位素(β 發射體和 α 發射體)、來源(反應器產生的同位素、發生器產生的同位素等)、治療應用(腫瘤學等)、載體類型(小分子配體、肽、單克隆抗體等)、最終用戶(醫院、放射性藥物和研究機構)劃分 - 行業趨勢和預測至 2032 年)
基於 α 和 β 放射體的放射性藥物市場規模
- 2024 年,北美基於 α 和 β 發射體的放射性藥物市場規模價值為3.3933 億美元 ,預計 到 2032 年將達到 7.8352 億美元,預測期內 複合年增長率為 11.1%。
- 市場成長主要得益於標靶 α 和 β 療法療效的提高
- 此外,個人化醫療中治療診斷的應用日益廣泛。這些因素正在加速基於α和β發射體的放射性藥物解決方案的普及,從而顯著促進該行業的成長。
基於α和β發射體的放射性藥物市場分析
- 基於α和β發射體的放射性藥物因其在標靶治療中的精確性而日益受到認可,特別是在腫瘤學和核子醫學領域,可提供有效的診斷和治療選擇,且副作用極小
- 全球癌症發生率的上升,以及人們對個人化醫療的認識不斷提高和放射性藥物技術的進步,正在推動北美對基於 α 和 β 發射體的放射性藥物的需求
- 美國在基於α和β發射體的放射性藥物市場佔有相當大的份額,佔2025年收入的約44.52%,這得益於先進的醫療基礎設施、廣泛的研發活動以及新型治療技術的早期採用
- 預計在預測期內,美國將成為基於 α 和 β 發射體的放射性藥物增長最快的市場,這得益於醫療基礎設施的擴大、癌症患病率的上升以及政府改善醫療狀況的舉措
- 預計到 2025 年,β 發射體領域將佔據市場主導地位,佔有 83.70% 的份額,這得益於其在靶向 α 療法 (TAT) 中的高效性、患者預後的改善,以及針對錒-225 和鐳-223 等 α 發射同位素用於癌症治療的研究日益增多
報告範圍以及基於 α 和 β 發射體的放射性藥物市場細分
|
屬性 |
基於α和β發射體的放射性藥物關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
北美洲
|
|
主要市場參與者 |
|
|
市場機會 |
|
|
加值資料資訊集 |
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。 |
基於α和β發射體的放射性藥物市場趨勢
“提高標靶 Alpha 和 Beta 療法的療效”
- 北美基於 α 和 β 發射體的放射性藥物市場的主要驅動力是靶向放射性核素療法在臨床上的應用日益增多,因為它們在治療神經內分泌腫瘤和轉移性去勢抵抗性前列腺癌 (mCRPC) 等晚期癌症方面已被證實有效
- 例如,根據NCBI於2023年5月發表的文章,核准的[177Lu]Lu-PSMA-617方案(每週期7.4 GBq,每6週給藥一次,最多6個週期)在實際應用中表現出了強大的安全性和抗腫瘤療效,且劑量(6-9.3 GBq)和治療週間隔(4-100)。這種穩定的臨床表現增強了醫師的信心,並加速了市場的採用。
- 像 Lu-177 這樣的放射性藥物,尤其是在肽受體放射性核素治療 (PRRT) 中使用時,已證明在神經內分泌腫瘤的治療中取得了顯著的成功,可將強效的 β 射線直接輸送到腫瘤部位,同時保留健康組織,從而改善治療效果並增加需求
- 鉺-225等α發射同位素的技術進步和臨床驗證進一步推動了市場的發展。鉺-225已被證明能夠有效靶向對傳統療法抗藥性的前列腺癌細胞,且副作用極小,療效顯著。
基於 α 和 β 放射體的放射性藥物市場動態
司機
“個人化醫療中診斷治療的應用日益廣泛”
- 镥-177(Lu-177)和铽-161(Tb-161)等α和β發射體放射性藥物的日益普及,是推動北美放射性藥物市場發展的主要驅動力。這些藥物將診斷影像與標靶治療結合在臨床工作流程中,提供精準、針對患者的個人化治療,從而改善療效並簡化治療計劃。
- 例如,2023年7月,NCBI發表的一篇綜述報告稱,基於镥-177的診療方案(例如,用於治療神經內分泌腫瘤的⁷⁷Lu-DOTATATE和用於治療前列腺癌的⁷⁷Lu-PSMA)的臨床應用正在不斷增加。 FDA批准這些藥物驗證了它們的安全性和有效性,加速了其應用,並凸顯了診斷-治療配對對的強大協同作用。
- 隨著臨床醫生尋求個人化癌症管理的可靠工具,腫瘤學家和核子醫學專家對治療診斷方法的工作流程效率、治療準確性和降低毒性的認識不斷提高,這推動了需求。
- 此外,隨著醫療保健系統面臨提高生存率和控製成本的越來越大的壓力,像 Lu-177 和 Tb-161 這樣的整合影像治療解決方案可以縮短治療時間,避免無效幹預,並提高生活質量,從而鞏固其價值主張
- 對精準腫瘤學的日益增長的偏好,加上對 Tb-149、Tb-152/155 和 Ac-225 等下一代同位素的持續研發,使得診斷治療放射性藥物成為現代癌症治療的基石和北美市場的主要增長引擎
克制/挑戰
“同位素半衰期短帶來的供應鏈和可擴展性挑戰”
- 等放射性核素的半衰期較短-212(約 10.6 小時),這給了物流和營運帶來重大障礙:生產必須在處理地點附近進行,運輸時間只有幾個小時,並且需要高度協調的「即時」供應鏈——這些因素共同限制了大規模生產和市場覆蓋範圍
- 例如,LEK Consulting 在 2025 年 4 月指出,Pb-212 的半衰期為 10.6 小時,這迫使其需要分散式、近患者生產和現場發電機基礎設施,從而限制了規模經濟,並使配送物流變得複雜
- 此外,提取 Pb-212(和其他短壽命同位素)所需的複雜發生器系統增加了監管合規性、輻射安全要求和資本支出的層層限制,使得醫院和放射性藥房難以廣泛部署
- 雖然緊湊型發電機、更快的淨化方法和區域生產中心的進步最終可以緩解這些壓力,但短壽命同位素的基本時間敏感性仍然是放射性藥物市場廣泛採用和增長的重大限制因素
基於 α 和 β 放射體的放射性藥物市場範圍
市場根據同位素、來源、治療應用、載體類型和最終用戶進行細分。
- 同位素
根據同位素類型,市場細分為β發射體和α發射體。預計到2025年,β發射體將佔據市場主導地位,市佔率達83.70%,這得益於鎔-177(Lu-177)和釔-90(Y-90)等同位素在臨床上廣泛用於治療神經內分泌腫瘤、肝癌和前列腺癌。 β發射體因其相對較長的半衰期、成熟的安全性以及與現有臨床工作流程的兼容性而受到青睞。
預計2025年至2032年間,β射線發射體市場將以11.1%的最快成長率成長,這得益於錒-225 (Ac-225) 和鉛-212 (Pb-212) 在晚期癌症治療中的應用不斷增加。 α射線發射體具有較高的線性能量轉移 (LET) 和更高的腫瘤殺傷效率,同時附帶損害最小,非常適合用於治療抗藥性和轉移性癌症。
- 來源
根據來源,市場可分為反應器生產同位素、發生器生產同位素和其他同位素。到2025年,由於镥-177和碘-131等關鍵β發射體供應量大且廣泛可用,反應器生產同位素將引領市場。
然而,預計反應器生產的同位素將成為成長最快的領域,這得益於對鉛-212和鐳-223等同位素的需求不斷增長,這些同位素需要分散式和近患者生產。現場發生器的興起也與人們對α療法和短壽命放射性藥物日益增長的興趣一致。
- 按治療應用
在治療應用方面,市場細分為腫瘤學和其他領域。 2025年,腫瘤學將佔據市場主導地位,因為放射性藥物在前列腺癌、神經內分泌腫瘤和淋巴瘤的標靶治療中發揮核心作用。 PSMA標靶療法和PRRT療法日益成功的鞏固了腫瘤學在該領域的領先地位。
腫瘤學領域包括心血管、內分泌和神經系統疾病,預計隨著新型放射性配體的開發和向非腫瘤適應症的擴展,腫瘤學領域將穩定成長。
- 依向量類型
根據載體類型,市場細分為小分子配體、勝肽、單株抗體和其他。預計到2025年,小分子配體將佔據最大的市場份額,因為它們具有快速的組織滲透性,並廣泛應用於PSMA和生長抑素標靶治療。
由於偶聯技術的進步及其提供更強的腫瘤選擇性、更長的循環時間和更高的結合效率,小分子配體在預測期內有望實現顯著增長。這些載體對於精準度至關重要的α發射體療法尤其重要。
- 按最終用戶
根據最終用戶,市場細分為醫院、放射藥房和研究機構。到2025年,醫院將佔據最大份額,這得益於患者獲得核醫學服務的管道不斷增加、診療科室的發展以及發達國家健全的報銷框架。
由於對放射性藥物(尤其是半衰期較短的藥物)集中和分散配製的需求不斷增長,放射性藥物市場預計將快速成長。研究機構將繼續在創新和臨床試驗中發揮重要作用,尤其是在铽-161和錒-225等下一代同位素方面。
基於 α 和 β 發射體的放射性藥物市場區域分析
- 美國仍然是基於 α 和 β 發射體的放射性藥物市場的全球領導者,預計 2025 年的複合年增長率為 12.8%。成長的動力來自該國癌症發病率高、標靶放射性核素療法的快速採用以及精準醫療的持續進步。
- 聯邦政府對核醫學研究的大力資助、強大的臨床試驗基礎設施以及領先生物技術公司、學術機構和能源部之間的戰略合作,正在加速治療同位素的創新。此外,錒-225和鎔-177等關鍵同位素的國內生產正在擴大規模,以確保供應鏈的韌性。這些動態使美國在全球α和β發射體放射性藥物的開發和商業化領域牢牢佔據主導地位。
加拿大基於 α 和 β 發射體的放射性藥物市場洞察
由於新的迴旋加速器和反應器升級支持了國內的 Lu-177 產量,加拿大實現了兩位數增長,而不斷擴大的門診治療中心網絡滿足了對精準腫瘤學日益增長的需求。
墨西哥基於 α 和 β 發射體的放射性藥物市場洞察
由於核醫學能力的不斷提升以及對先進癌症療法需求的不斷增長,墨西哥正逐漸成為北美放射性藥物市場中一個潛力巨大的參與者。該國不斷增加的公私合作投資、靶向α和β發射體技術的採用以及旨在提升國內同位素產量的舉措,正在推動市場發展勢頭。在墨西哥國家核子研究所 (ININ) 等機構的戰略支持下,墨西哥正將自身定位為一個重要的區域樞紐,提供便利且經濟高效的放射性藥物解決方案。
基於α和β發射體的放射性藥物市場份額
基於 α 和 β 發射體的放射性藥物市場主要由知名公司主導,包括:
- 諾華公司(瑞士)
- Eckert & Ziegler(德國)
- ITM Isotope Technologies Munich SE(德國)
- SHINE Technologies, LLC(美國)
- Actinium Pharmaceuticals, Inc.(美國)
- Alpha Tau Medical Ltd.(以色列)
- ARICEUM THERAPEUTICS(德國)
- 拜耳公司(德國)
- 鋦(美國)
- IONETIX公司(美國)
- Isotopia(以色列)
- Lantheus(美國)
- 禮來(美國)
- Niowave(美國)
- 核磁共振(美國)
- 歐安諾集團(巴黎)
- Telix Pharmaceuticals Limited(澳洲)
基於α和β發射體的放射性藥物市場的最新發展
- 2025年5月,ITM Isotope Technologies Munich SE與Radiopharm Theranostics宣布達成一項非載體添加镥-177(nca 177Lu)供應協議。該合作將支持Radiopharm基於镥-177療法的臨床開發,包括RAD 204、RAD 202和RV01,確保在正在進行和未來臨床試驗中,為實體腫瘤的靶向放射性藥物治療提供高品質的同位素。
- 2025年3月,FDA批准諾華公司的Pluvicto(Lu-177 vipivotide tetraxetan)用於治療PSMA陽性轉移性去勢抵抗性前列腺癌的早期治療,允許在一次ARPI後、化療前給藥。根據III期PSMAfore試驗的結果,Pluvicto降低了59%的疾病進展或死亡風險,使中位放射學無惡化存活期翻了一番,同時保持了良好的安全性,並顯著擴大了患者的治療可及性。
- 2025年3月,Eckert & Ziegler與AtomVie North America Radiopharma簽署了一份非載體添加镥-177(Theralugand)的北美供應協議。該合作將確保為AtomVie的CDMO放射性藥物業務提供穩定、高品質的镥-177供應,支持其在全球範圍內的早期至後期研發,並增強兩家公司在放射性藥物創新、法規合規性和以患者為中心的核醫學解決方案方面的能力。
- 2025年3月,Eckert & Ziegler與Actinium Pharmaceuticals簽署了高純度錒-225 (Ac-225) 供應協議。該合作旨在確保可靠的錒-225來源,以支持Actimab-A及其他針對急性髓系白血病(AML)和實體瘤的放射治療候選藥物的開發,增強Actinium Pharmaceuticals的臨床研發管線,並應對北美在精準放射性藥物治療領域面臨的同位素供應挑戰。
- 2024年5月,諾華公司宣布以10億美元的首付款和最高7.5億美元的里程碑付款收購Mariana Oncology。此次收購將增強諾華的放射配體療法(RLT)產品線,使其涵蓋針對實體瘤的臨床前資產,包括用於治療小細胞肺癌的錒系候選藥物MC-339,並增強其RLT的研究、供應和創新能力。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 END USER MARKET COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PIPELINE
4.4 SUPPLY CHAIN ECOSYSTEM
4.4.1 PROMINENT COMPANIES
4.4.2 SMALL & MEDIUM SIZE COMPANIES
4.4.3 END USERS
4.5 INDUSTRY INSIGHTS
4.5.1 MICRO AND MACRO ECONOMIC FACTORS
4.5.2 KEY PRICING STRATEGIES
4.6 MARKETED DRUG ANALYSIS
4.6.1 DRUG
4.6.1.1 BRAND NAME
4.6.1.2 GENERIC NAME
4.6.2 THERAPEUTIC INDICATION
4.6.3 PHARMACOLOGICAL CLASS OF THE DRUG
4.6.4 DRUG PRIMARY INDICATION
4.6.5 MARKET STATUS
4.6.6 MEDICATION TYPE
4.6.7 DRUG DOSAGE FORM
4.6.8 DOSAGES AVAILABILITY
4.6.9 PACKAGING TYPE
4.6.10 DRUG ROUTE OF ADMINISTRATION
4.6.11 DOSING FREQUENCY
4.6.12 DRUG INSIGHT
4.6.13 OVERVIEW OF DRUG DEVELOPMENT ACTIVITIES
4.6.13.1 FORECAST MARKET OUTLOOK
4.6.13.2 CROSS COMPETITION
4.6.13.3 THERAPEUTIC PORTFOLIO
4.6.13.4 CURRENT DEVELOPMENT SCENARIO
4.7 HEALTHCARE TARIFFS IMPACT ANALYSIS
4.7.1 OVERVIEW
4.7.2 TARIFF STRUCTURES
4.7.2.1 NORTH AMERICA VS. REGIONAL TARIFF STRUCTURES
4.7.2.2 UNITED STATES: MEDICARE/MEDICAID TARIFF POLICIES, CMS PRICING MODELS
4.7.2.3 EUROPEAN UNION: CROSS-BORDER TARIFF REGULATIONS, REIMBURSEMENT POLICIES
4.7.2.4 ASIA-PACIFIC: GOVERNMENT-IMPOSED TARIFFS ON IMPORTED MEDICAL PRODUCTS
4.7.2.5 EMERGING MARKETS: CHALLENGES IN TARIFF IMPLEMENTATION
4.7.3 PHARMACEUTICAL TARIFFS AND TRADE BARRIERS
4.7.3.1 IMPORT DUTIES ON PRESCRIPTION DRUGS VS. GENERICS
4.7.3.2 IMPACT ON DRUG AFFORDABILITY AND ACCESS
4.7.3.3 KEY TRADE AGREEMENTS AFFECTING PHARMACEUTICAL TARIFFS
4.8 IMPACT OF HEALTHCARE TARIFFS ON PROVIDERS AND PATIENTS
4.8.1.1 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.8.1.2 EFFECT ON PATIENT AFFORDABILITY AND INSURANCE COVERAGE
4.8.1.3 TARIFFS AND THEIR ROLE IN MEDICAL TOURISM
4.8.2 TRADE AGREEMENTS AND HEALTHCARE TARIFFS
4.8.2.1 WTO REGULATIONS ON HEALTHCARE TARIFFS
4.8.2.2 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.8.2.3 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
4.8.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.8.4 IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.9 EPIDEMIOLOGY OVERVIEW
4.9.1 INCIDENCE OF ALL CANCERS BY GENDER
4.9.2 TREATMENT RATE
4.9.3 MORTALITY RATE
4.9.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
4.9.5 PATIENT TREATMENT SUCCESS RATES
5 REGULATORY FRAMEWORK
5.1 REGULATORY FRAMEWORK OVERVIEW FOR THE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET
5.1.1 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
5.1.2 REGULATORY APPROVAL PATHWAYS
5.1.3 LICENSING AND REGISTRATION
5.1.4 POST-MARKETING SURVEILLANCE
5.1.5 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASED EFFICACY OF TARGETED ALPHA AND BETA THERAPIES
6.1.2 GROWING ADOPTION OF THERANOSTICS IN PERSONALIZED MEDICINE
6.1.3 RISING CLINICAL DEMAND FOR ALPHA-BASED RADIOTHERAPIES
6.1.4 RISING CHRONIC DISEASE BURDEN DRIVING RADIOPHARMACEUTICAL DEMAND
6.2 RESTRAINTS
6.2.1 SUPPLY CHAIN AND SCALABILITY CHALLENGES FROM SHORT ISOTOPE HALF-LIVES
6.2.2 STRINGENT REGULATORY LANDSCAPE LIMITING MARKET FLEXIBILITY
6.2.3 SAFETY AND EXPOSURE RISKS IN RADIOPHARMACEUTICAL USE
6.3 OPPORTUNITIES
6.3.1 SURGE IN R&D ACTIVITY EXPANDING RADIOPHARMACEUTICAL APPLICATIONS
6.3.2 EXPANSION OF LU-177-PSMA THERAPY IN PROSTATE CANCER TREATMENT
6.3.3 STRATEGIC COLLABORATIONS DRIVING RADIOPHARMACEUTICAL INNOVATION
6.4 CHALLENGES
6.4.1 HIGH COST OF DEVELOPMENT AND IMPLEMENTATION OF RADIOPHARMACEUTICALS
6.4.2 SHORTAGE OF SKILLED WORKFORCE IN NUCLEAR MEDICINE AND RADIOCHEMISTRY
7 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE
7.1 OVERVIEW
7.2 BETA EMITTERS
7.2.1 LUTETIUM-177
7.2.2 TERBIUM-161
7.3 ALPHA EMITTERS
7.3.1 ACTINIUM-225
7.3.2 LEAD -212
8 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY SOURCES
8.1 OVERVIEW
8.2 REACTOR-PRODUCED ISOTOPES
8.3 GENERATOR-PRODUCED ISOTOPES
8.4 OTHERS
9 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY THERAPEUTIC APPLICATION
9.1 OVERVIEW
9.2 ONCOLOGY
9.2.1 PROSTATE CANCER
9.2.2 NEUROENDOCRINE TUMORS
9.2.3 LIVER CANCER
9.2.4 BRAIN TUMORS
9.2.5 BREAST CANCER
9.2.6 LEUKEMIA
9.3 OTHERS
10 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY VECTOR TYPE
10.1 OVERVIEW
10.2 SMALL MOLECULE LIGANDS
10.3 PEPTIDES
10.4 MONOCLONAL ANTIBODIES
10.5 OTHERS
11 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 ONCOLOGY CENTERS
11.2.2 NUCLEAR MEDICINE DEPARTMENTS
11.3 RADIOPHARMACIES
11.4 RESEARCH INSTITUTES
12 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 NOVARTIS AG
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 ECKERT & ZIEGLER
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS
15.3 ITM ISOTOPE TECHNOLOGIES MUNICH SE
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 SHINE TECHNOLOGIES, LLC
15.4.1 COMPANY SNAPSHOT
15.4.2 COMPANY SHARE ANALYSIS
15.4.3 PRODUCT PORTFOLIO
15.4.4 RECENT DEVELOPMENT
15.5 ACTINIUM PHARMACEUTICALS, INC.
15.5.1 COMPANY SNAPSHOT
15.5.2 PIPELINE PRODUCT PORTFOLIO
15.5.3 RECENT DEVELOPMENTS
15.6 ALPHA TAU MEDICAL LTD.
15.6.1 COMPANY SNAPSHOT
15.6.2 PIPELINE PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 ARICEUM THERAPEUTICS
15.7.1 COMPANY SNAPSHOT
15.7.2 PIPELINE PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 BAYER AG
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PIPELINE PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 CURIUM
15.9.1 COMPANY SNAPSHOT
15.9.2 PIPELINE PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 IONETIX CORPORATION
15.10.1 COMPANY SNAPSHOT
15.10.2 PIPELINE PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 ISOTOPIA
15.11.1 COMPANY SNAPSHOT
15.11.2 PIPELINE PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 LANTHEUS
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PIPELINE PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 LILLY
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PIPELINE PRODUCT PORTFOLIO
15.14 NIOWAVE
15.14.1 COMPANY SNAPSHOT
15.14.2 PIPELINE PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 NMR
15.15.1 COMPANY SNAPSHOT
15.15.2 PIPELINE PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 ONCOINVENT
15.16.1 COMPANY SNAPSHOT
15.16.2 PIPELINE PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 ORANO GROUP
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PIPELINE PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 RADIOPHARM THERANOSTICS LIMITED
15.18.1 COMPANY SNAPSHOT
15.18.2 PIPELINE PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
15.19 TELIX PHARMACEUTICALS LIMITED
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PIPELINE PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENT
15.2 TERTHERA
15.20.1 COMPANY SNAPSHOT
15.20.2 PIPELINE PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
15.20.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
表格列表
TABLE 1 NORTH AMERICA CLINICAL TRIAL MARKET FOR NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET
TABLE 5 PENETRATION AND GROWTH PROSPECT MAPPING
TABLE 6 INCIDENCE OF CANCER BY GENDER
TABLE 7 CANCER MORTALITY RATE
TABLE 8 CANCER TREATMENT SUCCESS RATE
TABLE 9 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE, 2018-2032 (USD THOUSAND)
TABLE 10 NORTH AMERICA BETA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 11 NORTH AMERICA BETA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 12 NORTH AMERICA ALPHA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 NORTH AMERICA ALPHA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY SOURCES, 2018-2032 (USD THOUSAND)
TABLE 15 NORTH AMERICA REACTOR-PRODUCED ISOTOPES IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 16 NORTH AMERICA GENERATOR-PRODUCED ISOTOPES IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 17 NORTH AMERICA OTHERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY THERAPEUTIC APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 19 NORTH AMERICA ONCOLOGY IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 NORTH AMERICA ONCOLOGY IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 21 NORTH AMERICA OTHERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY VECTOR TYPE, 2018-2032 (USD THOUSAND)
TABLE 23 NORTH AMERICA SMALL MOLECULE LIGANDS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 NORTH AMERICA PEPTIDES IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 NORTH AMERICA MONOCLONAL ANTIBODIES IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 26 NORTH AMERICA OTHERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 28 NORTH AMERICA HOSPITALS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 29 NORTH AMERICA HOSPITALS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 NORTH AMERICA RADIOPHARMACIES IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 31 NORTH AMERICA RESEARCH INSTITUTES IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 32 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 33 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE, 2018-2032 (USD THOUSAND)
TABLE 34 NORTH AMERICA BETA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 NORTH AMERICA ALPHA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY SOURCES, 2018-2032 (USD THOUSAND)
TABLE 37 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY THERAPEUTIC APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 38 NORTH AMERICA ONCOLOGY IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 39 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY VECTOR TYPE, 2018-2032 (USD THOUSAND)
TABLE 40 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 41 NORTH AMERICA HOSPITALS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 U.S. ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE, 2018-2032 (USD THOUSAND)
TABLE 43 U.S. BETA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 U.S. ALPHA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 U.S. ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY SOURCES, 2018-2032 (USD THOUSAND)
TABLE 46 U.S. ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY THERAPEUTIC APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 47 U.S. ONCOLOGY IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 48 U.S. ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY VECTOR TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 U.S. ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 50 U.S. HOSPITALS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 CANADA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE, 2018-2032 (USD THOUSAND)
TABLE 52 CANADA BETA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 CANADA ALPHA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 CANADA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY SOURCES, 2018-2032 (USD THOUSAND)
TABLE 55 CANADA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY THERAPEUTIC APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 56 CANADA ONCOLOGY IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 CANADA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY VECTOR TYPE, 2018-2032 (USD THOUSAND)
TABLE 58 CANADA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 59 CANADA HOSPITALS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 MEXICO ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE, 2018-2032 (USD THOUSAND)
TABLE 61 MEXICO BETA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 MEXICO ALPHA EMITTERS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 MEXICO ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY SOURCES, 2018-2032 (USD THOUSAND)
TABLE 64 MEXICO ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY THERAPEUTIC APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 65 MEXICO ONCOLOGY IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 66 MEXICO ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY VECTOR TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 MEXICO ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 68 MEXICO HOSPITALS IN ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
图片列表
FIGURE 1 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: MULTIVARIATE MODELLING
FIGURE 7 CURVE LINE CHART, BY ISOTOPE
FIGURE 8 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 9 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: DBMR MARKET POSITION GRID
FIGURE 10 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: END USER MARKET COVERAGE GRID
FIGURE 12 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: SEGMENTATION
FIGURE 13 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 TWO SEGMENTS COMPRISE THE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET, BY ISOTOPE 2024
FIGURE 16 INCREASED EFFICACY OF TARGETED ALPHA AND BETA THERAPIES IS EXPECTED TO DRIVE THE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET IN THE FORECAST PERIOD
FIGURE 17 BETA EMITTERS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET IN 2025 & 2032
FIGURE 18 INCIDENCE BY CANCER SITE
FIGURE 19 CANCER MORTALITY RATE WITH CANCER SITE
FIGURE 20 DROC ANALYSIS
FIGURE 21 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY ISOTOPE, 2024
FIGURE 22 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY ISOTOPE, 2025 TO 2032 (USD THOUSAND)
FIGURE 23 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY ISOTOPE, CAGR (2025-2032)
FIGURE 24 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY ISOTOPE, LIFELINE CURVE
FIGURE 25 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY SOURCES, 2024
FIGURE 26 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY SOURCES, 2025 TO 2032 (USD THOUSAND)
FIGURE 27 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY SOURCES, CAGR (2025-2032)
FIGURE 28 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY SOURCES, LIFELINE CURVE
FIGURE 29 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY THERAPEUTIC APPLICATION, 2024
FIGURE 30 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY THERAPEUTIC APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 31 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY THERAPEUTIC APPLICATION, CAGR (2025-2032)
FIGURE 32 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY THERAPEUTIC APPLICATION, LIFELINE CURVE
FIGURE 33 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY VECTOR TYPE, 2024
FIGURE 34 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY VECTOR TYPE, 2025 TO 2032 (USD THOUSAND)
FIGURE 35 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY VECTOR TYPE, CAGR (2025-2032)
FIGURE 36 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY VECTOR TYPE, LIFELINE CURVE
FIGURE 37 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY END USER, 2024
FIGURE 38 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 39 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY END USER, CAGR (2025-2032)
FIGURE 40 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: BY END USER, LIFELINE CURVE
FIGURE 41 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: SNAPSHOT (2024)
FIGURE 42 NORTH AMERICA ALPHA AND BETA EMITTERS BASED RADIOPHARMACEUTICALS MARKET: COMPANY SHARE 2024 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。