North America Biopsy Devices Market
市场规模(十亿美元)
CAGR :
% 
 USD
1.80 Billion
USD
3.60 Billion
2024
2032
USD
1.80 Billion
USD
3.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.80 Billion | |
| USD 3.60 Billion | |
|
|
|
|
North America Biopsy Devices Market Segmentation, By Product (Needle-Based Biopsy Instruments, Procedure Trays, Localization Wires and other Products), Application (Breast Biopsy, Lung Biopsy, Colorectal Biopsy, Prostate Biopsy and Other Applications), Guidance Technique (Ultrasound-Guided Biopsy, Stereotactic-Guided Biopsy, MRI-Guided Biopsy and Other Guidance Techniques), End User (Hospitals, Academic and Research Institutes, Diagnostic and Imaging Centers) – Industry Trends and Forecast to 2032
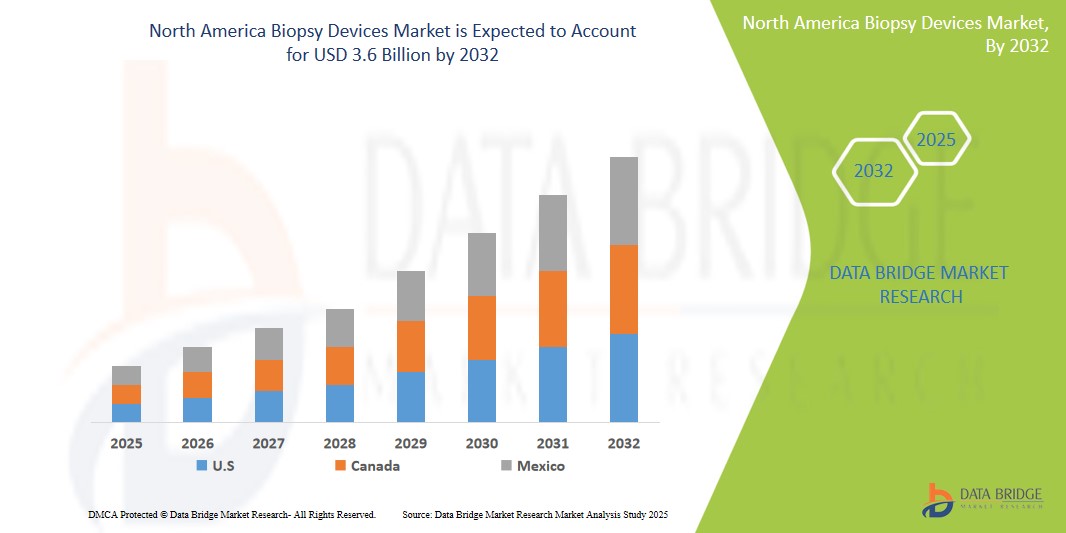
North America Biopsy Devices Market Size
- The North America Biopsy Devices Market was valued atUSD1.8 Billion in 2024 and is expected to reachUSD3.6 Billion by 2032
- Drivers of the North America Biopsy Devices Market include the rising prevalence of cancer and other chronic diseases, which necessitate accurate diagnostic tools for early detection and treatment. Additionally, advancements in biopsy technologies, such as minimally invasive techniques and imaging guidance, are enhancing procedural efficiency and patient outcomes. The increasing emphasis on personalized medicine and the growing number of diagnostic and outpatient procedures further propel market growth.
North America Biopsy Devices Market Analysis
- The North America Biopsy Devices Market is experiencing robust growth due to the rising incidence of cancer and an increasing focus on early diagnosis and intervention. The aging population and a greater awareness of health issues contribute to the demand for advanced biopsy technologies.
- Innovations in biopsy techniques, including image-guided biopsies and minimally invasive procedures, are driving the market. These advancements improve accuracy, reduce patient discomfort, and shorten recovery times, making them more appealing to healthcare providers and patients alike.
- Favorable regulatory environments and reimbursement policies in the U.S. and Canada support the adoption of biopsy devices. This environment encourages manufacturers to develop and market advanced diagnostic tools, further boosting market growth.
- The market features several major players, including companies like Bard, Hologic, and Devicor Medical Products, which are continually innovating and expanding their product offerings. Competitive strategies often involve mergers, acquisitions, and partnerships to enhance market presence and technological capabilities.
Report Scope andNorth America Biopsy Devices MarketSegmentation
|
Attributes |
North America Biopsy Devices MarketInsights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Biopsy Devices MarketTrends
“Growing Adoption of Image-Guided Biopsy Techniques”
- Image-guided biopsy techniques, such as ultrasound, CT, and MRI-guided procedures, enhance the accuracy of sample collection, allowing for more precise targeting of lesions. This precision is crucial for effective diagnosis, particularly in complex cases, leading to better patient outcomes.
- Patients and healthcare providers increasingly favor minimally invasive techniques that reduce recovery time and postoperative complications. Image-guided biopsies fulfill this demand, offering safer alternatives to traditional surgical biopsies with shorter hospital stays and quicker return to daily activities.
- Continuous advancements in imaging technologies are facilitating the integration of high-resolution imaging with biopsy devices, enabling more effective and streamlined procedures. As these technologies evolve, their application in biopsy devices is expected to become even more widespread, driving market growth and innovation.
North America Biopsy Devices Market Dynamics
Driver
“Increasing Incidence of Cancer”
- The incidence of various types of cancer, including breast, lung, and prostate cancer, is increasing in North America due to factors such as an aging population, lifestyle changes, and environmental exposures. This rise in cancer cases boosts the demand for diagnostic devices, including biopsy devices, which are essential for accurate diagnosis and treatment planning.
- Early and accurate detection of cancer significantly improves treatment outcomes and increases survival rates. As healthcare providers emphasize preventive care and early diagnosis, the demand for biopsy devices that facilitate timely and effective cancer detection has surged, driving market growth.
- Continuous innovation in biopsy technology, including the development of less invasive and more precise methods, enhances the appeal of biopsy devices. These advancements not only improve diagnostic capabilities but also encourage healthcare providers to adopt biopsies as a standard practice in cancer diagnosis, further fueling market demand.
Opportunity
“Expanding Applications of Biopsy Devices in Oncology”
- The rising prevalence of various cancers, particularly breast, lung, and colorectal cancers, presents a significant opportunity for the biopsy devices market. Early and accurate diagnostic techniques, including biopsies, are essential for effective treatment planning and management, leading to greater demand for advanced biopsy solutions.
- As the focus on personalized medicine grows, there is an increasing need for precise tumor characterization through biopsy techniques. This enables tailored treatments based on the specific genetic and molecular profile of a patient’s cancer, expanding the role of biopsy devices in oncology and enhancing their market potential.
- The ongoing development of novel biopsy technologies, such as liquid biopsies and advanced imaging-guided procedures, offers new avenues for market growth. These innovations provide less invasive options for obtaining tissue samples and enhance diagnostic accuracy, making biopsy devices more appealing to both providers and patients.
Restraint/Challenge
“Regulatory and Reimbursement Issues”
- The process for obtaining regulatory approval for new biopsy devices in North America can be lengthy and complex. Manufacturers must navigate through rigorous standards set by organizations like the FDA, which can delay the introduction of innovative products to the market and increase development costs.
- Securing reimbursement from insurance providers for biopsy procedures can be challenging. Variability in coverage policies and reimbursement rates across different insurers can deter healthcare providers from adopting newer biopsy technologies, limiting their market potential.
- High costs associated with advanced biopsy devices may restrict their accessibility, especially in lower-resource healthcare settings. Hospitals and clinics may be hesitant to invest in expensive technologies without assurance of adequate reimbursement, further complicating market penetration.
North America Biopsy Devices Market Scope
The market is segmented on the basis of type, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Product |
Needle-Based Biopsy Instruments, Procedure Trays, Localization Wires and other Products |
|
ByApplication |
Breast Biopsy, Lung Biopsy, Colorectal Biopsy, Prostate Biopsy and Other Applications |
|
ByGuidance Technique |
Ultrasound-Guided Biopsy, Stereotactic-Guided Biopsy, MRI-Guided Biopsy and Other Guidance Techniques |
|
By End User |
Hospitals, Academic and Research Institutes, Diagnostic and Imaging Centers) |
North America Biopsy Devices Market Analysis
“U.S. is the Dominant with around 38% market share in the North America Biopsy Devices Market”
- The United States boasts a highly developed healthcare system with sophisticated medical facilities and advanced technological capabilities. This infrastructure supports the adoption of cutting-edge biopsy devices, making the U.S. a leader in the market. Hospitals and clinics in the U.S. are equipped with the latest imaging technologies and biopsy tools, which enhances the precision and efficiency of diagnostic procedures.
- The U.S. is home to a large number of medical device manufacturers and biotech companies that heavily invest in research and development. This commitment leads to continuous innovation in biopsy technology, including the development of minimally invasive techniques and advanced imaging-guided devices. The financial resources available for R&D contribute to the growth and dominance of the U.S. in the biopsy market.
- The U.S. healthcare system offers a relatively robust reimbursement framework for biopsy procedures, facilitating greater access to these services for patients. Insurance companies and government programs often cover advanced diagnostic procedures, encouraging healthcare providers to adopt the latest biopsy technologies. This support creates a conducive environment for market expansion and places the U.S. at the forefront of the North American Biopsy Devices Market.
“U.S. is Projected to Register the Highest Growth Ratein theNorth America Biopsy Devices Market”
- The U.S. is home to a robust ecosystem of research and development in the healthcare sector, leading to significant innovations in biopsy devices. Continuous advancements in imaging technologies and minimally invasive techniques are driving the adoption of sophisticated biopsy solutions, thus fueling market growth.
- The rising prevalence of cancer and other chronic diseases in the U.S. has led to a higher demand for diagnostic procedures, including biopsies. As early detection of cancer becomes a priority in healthcare, the need for advanced biopsy devices is expected to rise, contributing to the market’s growth in the region.
North America Biopsy Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- BD (Becton, Dickinson and Company)
- Hologic, Inc.
- Danaher Corporation ( (including brands like Leica Biosystems)
- Argon Medical Devices (
- Cook Medical
- AbbVie Inc. (Allergan)
- Bayer AGss
- The Cooper Companies, Inc.
- DKT International
- EUROGINE S.L.
- Pregna International Limited
- Prosan International BV
- SMB Corporation of India
- Melbea AG
- OCON Medical Ltd.
- Mylan N.V. (Viatris)
- Teva Pharmaceutical Industries Ltd.
- Merck & Co., Inc.
- Pfizer Inc.
- HLL Lifecare Limited
Latest Developments in North America Biopsy Devices Market
- In 2024, Hologic, Inc. launched its Breast Biopsy Suite, integrating advanced imaging and biopsy tools for more precise diagnostics.
- In 2023, BD (Becton, Dickinson and Company) introduced a next-generation core biopsy needle with enhanced tissue sample accuracy.
- In 2023, Argon Medical Devices released a new vacuum-assisted biopsy system for minimally invasive procedures.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。