North America Postpartum Hemorrhage Treatment Devices Market
市场规模(十亿美元)
CAGR :
% 
 USD
396.17 Million
USD
546.68 Million
2024
2032
USD
396.17 Million
USD
546.68 Million
2024
2032
| 2025 –2032 | |
| USD 396.17 Million | |
| USD 546.68 Million | |
|
|
|
|
北美產後出血治療設備市場細分,按類型(子宮球囊填塞、Uniject 預充式註射系統、非氣動防休克服、真空誘發的止血設備等)、病症(產後大出血(超過 1000 毫升)、產後小出血(500-1000 毫升)、產後大出血、繼發性大出血(2000 毫升) PPH)、最終用戶(醫院、婦產中心、專科診所、家庭護理機構等)、分銷管道(直接投標、零售等)- 行業趨勢和預測到 2032 年
產後出血治療設備市場規模
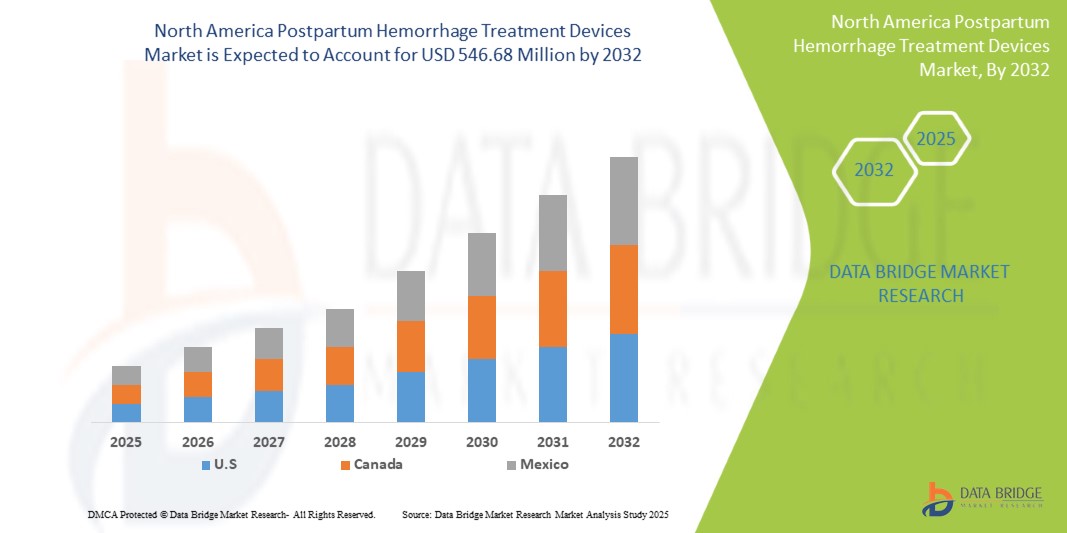
- 2024 年北美產後出血治療設備市場價值為3.9617 億美元,預計到 2032 年將達到 5.4668 億美元
- 在 2025 年至 2032 年的預測期內,市場可能以4.1% 的複合年增長率成長,主要受預期推出的新治療設備的推動
- 這種增長是由產後出血發病率上升等因素推動的,特別是在發展中國家,推動了對產後出血治療設備的需求
產後出血治療設備分析
- 由於剖腹產率上升和分娩併發症等因素,產後出血(PPH)的發生率不斷上升,導致對有效治療設備的需求不斷增長
- 此外,醫療技術的創新,包括先進的子宮填塞裝置、止血劑和微創手術技術的開發,正在顯著改善患者的治療效果並推動市場成長
- 例如,PPH 治療設備市場呈現出明顯的地理差異,由於醫療保健投資的增加和對改善產婦保健基礎設施的重視程度的提高,發展中地區的市場出現了顯著增長
- 總而言之,不斷增長的臨床需求和持續的技術進步為製造商和醫療保健提供者創造了強勁的成長機會,最終支持了全球降低孕產婦死亡率和加強產後護理的努力。
報告範圍及產後出血治療設備細分
|
屬性 |
產後出血治療設備關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
北美洲
|
|
主要市場參與者 |
|
|
市場機會 |
|
|
加值資料資訊集 |
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括進出口分析、生產能力概覽、生產消費分析、價格趨勢分析、氣候變遷情景、供應鏈分析、價值鏈分析、原材料/消耗品概覽、供應商選擇標準、PESTLE 分析、波特分析和監管框架。 |
產後出血治療設備市場 趨勢
“子宮球囊填塞裝置的採用率不斷提高”
- 子宮球囊填塞裝置旨在透過對子宮壁施加直接壓力來控制產後出血,促進止血
- 它們在臨床環境中的有效性已被廣泛記錄,這增加了醫療保健提供者使用這些設備作為管理產後出血的一線幹預措施的信心,從而推動了它們的採用
- 例如, 醫療保健界一直在齊心協力改善產後出血管理方面的培訓和教育。這包括關於子宮球囊填塞技術使用的專門訓練。醫療保健專業人員對及時幹預重要性的認識不斷提高,使得這些設備在已開發地區和發展中地區都得到了更廣泛的接受和使用。
- 子宮球囊填塞裝置正越來越多地被納入綜合產婦照護方案,尤其是在急診產科。這種整合得到了世界衛生組織等組織以及各種孕產婦健康倡議的指導方針的支持,這些倡議提倡循證實踐,以有效預防和管理產後出血 (PPH)。
- 這些設備與臨床指南的一致性促進了它們在醫院和分娩中心的廣泛採用
產後出血治療設備市場動態
司機
“產後出血發生率上升”
- 產後出血(PPH)發生率的不斷上升使得北美市場對有效治療設備的需求日益迫切
- 人們對孕產婦健康問題的認識不斷提高,對高風險族群的識別也更加準確,導致對創新解決方案的需求激增
- 隨著醫療保健系統將產婦安全放在首位,用於預防或控制產後出血的先進醫療設備的採用正在增加,包括子宮壓迫裝置和止血劑
例如,
- 2020年5月,根據NCBI的數據,產後出血(PPH)是全球孕產婦死亡的主要原因。在美國,PPH發生率上升了26%。這一驚人的病例成長凸顯了解決PPH問題的重要性,並促使人們增加對創新治療方案和技術的投資,以預防和管理這種可能危及生命的疾病。
- 2024年8月,世界衛生組織指出,產後出血(PPH)通常定義為產後24小時內失血500毫升或以上,是全球孕產婦死亡的主要原因。每年有數百萬女性受到影響,佔北美已通報孕產婦死亡總數的20%以上。
- 此外,剖腹產率上升和妊娠併發症發生率上升等因素也加劇了改善產後出血 (PPH) 管理解決方案的需求。醫療保健機構正致力於制定和實施標準化方案,以有效應對產後出血 (PPH),從而推動市場成長。技術創新催生了更有效率的新型設備,進一步增強了治療方案,並在全球範圍內改善了產婦預後。
機會
“PPH治療設備正確使用的訓練和教育計劃”
- 制定關於正確使用 PPH(產後出血)治療設備的全面培訓和教育計劃,為加強產婦保健服務提供了寶貴的機會
- 這些計畫可以針對不同級別的醫護人員進行量身定制,從社區衛生工作者到熟練的助產士和醫院工作人員,確保所有參與產婦護理的人員都具備必要的知識和實踐經驗
- 專注於實踐演示、模擬和基於案例的學習,可以顯著提高器械操作和及時幹預的能力。此外,持續的進修課程和數位學習平台有助於維持較高的能力水平,從而改善患者預後並降低孕產婦死亡率。
例如,
- 2025年4月,MDPI強調虛擬實境(VR)技術在產科教育領域將帶來巨大的進步,它符合現代學習習慣,並能提升臨床準備水準。將其融入培訓項目,可以透過提供沉浸式、以患者為中心的學習體驗來改善產後出血(PPH)的管理,從而增強產婦護理的技術技能和情緒準備。
- 2024年12月,《婦產科雜誌》的研究結果表明,結合案例式學習 (CBL) 和問題式學習 (PBL) 並結合情境模擬的方法,顯著提高了經驗豐富的產科醫護人員的臨床思維、操作技能和產後出血 (PPH) 知識。該方法在農村地區被證明是有效的,增強了受訓人員的自信心,並有望降低嚴重產後出血病例數和轉運率。
- 實施針對性培訓和教育計劃,正確使用產後出血 (PPH) 治療設備,對於改善全球孕產婦健康至關重要。
- 來自不同研究的證據強調了基於模擬的學習、虛擬實境以及基於案例和基於問題的綜合方法等方法的有效性。
克制/挑戰
“產後出血治療的副作用”
- 產後出血 (PPH) 治療設備相關的副作用可能會阻礙醫療保健提供者完全接受這些技術
- 由於擔心感染、子宮穿孔或材料不良反應等併發症,臨床醫生經常權衡使用這些設備的益處與相關風險
- 因此,一些醫療保健專業人員可能更傾向於保守的管理方法或傳統方法,而不是新的干預措施,這限制了旨在有效治療 PPH 的創新治療方法的採用
例如,
- 2021年7月,《卡格藥理學》(Karger Pharmacology)指出,注射前列腺素(PG)用於治療產後出血(PPH)可有效減少失血,但可能引發心血管或呼吸系統副作用。 《卡格藥理學》強調,注射用前列腺素(PG)治療產後出血(PPH)可能有心血管和呼吸系統副作用,這可能會增加醫護人員的安全顧慮,從而降低其使用安全性,並對北美PPH治療器械市場造成負面影響。
- 2024年,MDPI宣布,用於治療產後出血的米索前列醇可能引發更多副作用,如發燒、發冷、噁心和嘔吐。這可能會對北美產後出血 (PPH) 治療設備市場產生負面影響,降低醫療服務提供者對藥物選擇的信心,並促使人們轉向尋求更可靠、更有效的 PPH 治療設備。
- 此外,潛在的副作用可能會對患者對產後出血 (PPH) 治療方案的信心產生負面影響。如果患者被告知某些器械可能有併發症的風險,他們可能會不願意尋求更先進的醫療幹預措施,而是選擇尋求其他解決方案或延遲治療。
- 患者的這種擔憂可能會導致這些設備的接受度和使用率降低,最終阻礙市場成長,並降低產後出血管理策略在改善產婦健康結果方面的整體有效性
北美產後出血治療設備市場範圍
北美產後出血治療設備市場根據類型、病情、患者類型、最終用戶和分銷管道分為五個顯著的部分。
|
分割 |
細分 |
|
按類型 |
|
|
按條件 |
|
|
依患者類型 |
|
|
按最終用戶
|
|
|
按分銷管道 |
|
北美產後出血治療設備市場區域分析
“美國是北美產後出血治療設備市場的主導國家”
- 美國擁有高度先進的醫療基礎設施,包括先進的醫療設備、尖端的醫療技術以及完善的醫療服務網絡。這些基礎設施使得產後出血(PPH)的診斷和治療更加有效,從而促進了創新治療設備的快速普及。
- 美國的剖腹產率相對較高,這是產後出血的重要風險因子。因此,對有效的產後出血 (PPH) 治療方案的需求日益增加。手術分娩的普及率不斷上升,加上人們對產婦安全的日益關注,推動了北美對先進 PPH 治療設備的需求。
- 美國FDA等監管框架促進了新醫療器材的審批和上市。此外,政府和私部門對孕產婦保健研發的大量投入也促進了產後出血(PPH)治療方案的創新。這種環境鼓勵有效器材的持續改進和推廣,進一步鞏固了該地區在市場上的領導地位。
“預計美國將實現市場最高複合年增長率”
- 美國擁有強大的產婦健康意識計劃和更好的產前護理,從而可以早期發現和主動管理產後出血,從而推動對先進治療設備的需求。
- 越來越多的女性在 35 歲以上生育,這會增加產後出血的風險,從而增加對球囊填塞物、非氣動防休克服 (NASG) 和手術設備等預防和治療設備的需求。
產後出血治療設備市場佔有率
市場競爭格局按競爭對手提供詳細資料。詳細資訊包括公司概況、公司財務狀況、收入、市場潛力、研發投入、新市場計劃、歐洲業務、生產基地和設施、生產能力、公司優勢和劣勢、產品發布、產品寬度和廣度以及應用主導地位。以上提供的數據點僅與公司在市場中的重點相關。
市場中主要的市場領導者有:
- BD(美國)
- Organon集團公司(美國)
- Laborie(美國)
- 庫柏公司(美國)
- 貝爾蒙特醫療技術公司(美國)
- 凝血治療學(美國)
- Sterimed集團(印度)
- RevMedx(美國)
- Maternova Inc.(美國)
- Sinapi Biomedical(南非)
- 猶他醫療產品公司(美國)
- Angiplast Private Limited(印度)
- Krishco Medical Products Pvt. Ltd.(印度)
- 3rd Stone Design(美國)
- Advin Health Care(印度)
產後出血治療設備的最新進展
- 2025年4月,Organon從百健(Biogen)手中收購了托珠單抗(ACTEMRA)的生物相似藥TOFIDENCE在美國的權益。此舉增強了Organon在免疫學領域的生物相似藥產品組合,拓展了關節炎和新冠肺炎的治療選擇。 TOFIDENCE於2024年5月上市,可治療多種發炎疾病,並支持Organon生物相似藥業務的成長,具有巨大的市場潛力。
- 2023年11月,庫博公司以3億美元收購了庫克醫療的部分資產,增強了庫博醫療旗下女性健康和外科產品組合。此次收購涵蓋Bakri球囊和多普勒監視器等產品。預計此次收購將提升庫博在2024年的營收和獲利,並鞏固其在北美生育和婦科醫療保健領域的地位。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 MARKET END USER COVERAGE GRID
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 INDUSTRY INSIGHTS
4.3.1 MICRO AND MACROECONOMIC FACTORS
4.3.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.3.3 KEY PRICING STRATEGIES
4.4 COST ANALYSIS BREAKDOWN
4.5 TECHNOLOGY ROADMAP
4.6 VALUE CHAIN ANALYSIS
4.7 OPPORTUNITY MAP ANALYSIS
4.8 HEALTHCARE ECONOMY
4.9 REIMBURSEMENT FRAMEWORK
4.1 TARIFFS AND ITS IMPACT ON THE MARKET
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 NORTH AMERICA VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE
6.1.2 ONGOING TECHNOLOGICAL ADVANCEMENTS FOR POSTPARTUM HEMORRHAGE TREATMENTS
6.1.3 RISING BIRTH RATES ASSOCIATED WITH AN INCREASE IN THE NUMBER OF POSTPARTUM HEMORRHAGE
6.1.4 REGULATORY SUPPORT AND APPROVALS ASSOCIATED WITH THE TREATMENT DEVICES
6.2 RESTRAINTS
6.2.1 SIDE EFFECTS ASSOCIATED WITH THE POSTPARTUM HEMORRHAGE TREATMENT
6.2.2 LIMITED RESEARCH AND DEVELOPMENT FOR PPH TREATMENT
6.3 OPPORTUNITIES
6.3.1 TRAINING AND EDUCATIONAL PROGRAMS FOR PROPER USE OF PPH TREATMENT DEVICES
6.3.2 SUPPORT FROM GOVERNMENTAL AND NON-GOVERNMENTAL ORGANIZATIONS IN PPH DEVICE ADOPTION
6.3.3 TELEMEDICINE INTEGRATION TO ENHANCE POSTPARTUM HEMORRHAGE DEVICE USE
6.4 CHALLENGES
6.4.1 ENVIRONMENTAL IMPACT AND DISPOSAL ISSUES OF SINGLE-USE PPH DEVICES
6.4.2 STERILITY CHALLENGES AND INFECTION RISKS IN PPH DEVICES
7 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE
7.1 OVERVIEW
7.2 UTERINE BALLOON TAMPONADE
7.2.1 BAKRI BALLOON
7.2.1.1 BAKRI POSTPARTUM BALLOON
7.2.1.2 BAKRI POSTPARTUM BALLOON WITH RAPID INSTILLATION COMPONENTS
7.2.2 FOLEY CATHETER
7.2.2.1 STANDARD FOLEY CATHETER
7.2.2.2 CONDOM-LOADED FOLEY CATHETER
7.3 UNIJECT PREFILLED INJECTION SYSTEM
7.3.1 OXYTOCIN-BASED INJECTION SYSTEM
7.3.2 CARBETOCIN-BASED INJECTION SYSTEM
7.4 NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1 STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1.1 MEDIUM
7.4.1.2 LARGE
7.4.2 MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.2.1 MEDIUM
7.4.2.2 LARGE
7.5 VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES
7.5.1 JADA SYSTEM
7.5.2 OTHERS
7.6 OTHERS
7.6.1 COMPRESSION DEVICES
7.6.1.1 B-LYNCH
7.6.1.2 HAYMAN
7.6.1.3 OTHER
7.6.2 UTERINE ARTERY LIGATION PRODUCTS
7.6.3 OTHERS
8 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE
8.1 OVERVIEW
8.2 PRIMARY PPH
8.3 SECONDARY PPH
9 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION
9.1 OVERVIEW
9.2 MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML)
9.3 MINOR POSTPARTUM HEMORRHAGE (500-1000 ML)
9.4 MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE)
9.5 SECONDARY POSTPARTUM HEMORRHAGE
10 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 RETAIL SALES
10.3.1 OFFLINE SALES
10.3.2 ONLINE SALES
10.4 OTHERS
11 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 PUBLIC HOSPITALS
11.2.1.1 TIER 2
11.2.1.2 TIER 3
11.2.1.3 TIER 1
11.2.2 PRIVATE HOSPITALS
11.2.2.1 TIER 2
11.2.2.2 TIER 3
11.2.2.3 TIER 1
11.3 MATERNITY CENTERS
11.4 SPECIALTY CLINICS
11.5 HOME CARE SETTINGS
11.6 OTHERS
12 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 SOLUTION PORTFOLIO
15.1.5 RECENT NEWS
15.2 ORGANON GROUP OF COMPANIES
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS/NEWS
15.3 LABORIE
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 COOPERCOMPANIES
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 BELMONT MEDICAL TECHNOLOGIES
15.5.1 COMPANY SNAPSHOT
15.5.2 COMPANY SHARE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENT
15.6 ADVIN HEALTH CARE
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 ANGIPLAST PRIVATE LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 COAGULANT THERAPEUTICS
15.8.1 COMPANY SNAPSHOT
15.8.2 PIPELINE PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 KRISHCO MEDICAL PRODUCTS PVT. LTD
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 MATERNOVA INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 REVMEDX
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 3RD STONE DESIGN
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENTS
15.13 STERIMED GROUP
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.14 UTAH MEDICAL PRODUCTS, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SINAPI BIOMEDICAL
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
表格列表
TABLE 1 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 NORTH AMERICA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 NORTH AMERICA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 NORTH AMERICA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 NORTH AMERICA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 NORTH AMERICA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 NORTH AMERICA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 NORTH AMERICA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 NORTH AMERICA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 10 NORTH AMERICA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 11 NORTH AMERICA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 12 NORTH AMERICA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 NORTH AMERICA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 NORTH AMERICA OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 NORTH AMERICA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 16 NORTH AMERICA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 NORTH AMERICA PRIMARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 NORTH AMERICA SECONDARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 21 NORTH AMERICA MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 NORTH AMERICA MINOR POSTPARTUM HEMORRHAGE (500-1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 NORTH AMERICA MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 NORTH AMERICA SECONDARY POSTPARTUM HEMORRHAGE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 NORTH AMERICA DIRECT TENDER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 NORTH AMERICA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 NORTH AMERICA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 NORTH AMERICA OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 30 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 31 NORTH AMERICA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 32 NORTH AMERICA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 NORTH AMERICA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 NORTH AMERICA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 NORTH AMERICA MATERNITY CENTERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 NORTH AMERICA SPECIALTY CLINICS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 37 NORTH AMERICA HOME CARE SETTINGS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 NORTH AMERICA OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 40 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 NORTH AMERICA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 NORTH AMERICA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 NORTH AMERICA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 NORTH AMERICA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 NORTH AMERICA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 NORTH AMERICA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 47 NORTH AMERICA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 48 NORTH AMERICA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 NORTH AMERICA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 NORTH AMERICA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 52 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 54 NORTH AMERICA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 NORTH AMERICA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 NORTH AMERICA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 58 NORTH AMERICA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 59 U.S. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 U.S. UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 U.S. BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 U.S. FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 U.S. UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 U.S. NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 U.S. STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 66 U.S. MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 67 U.S. VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 U.S. OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 U.S. COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 U.S. NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 71 U.S. NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 U.S. NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 U.S. HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 U.S. PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 U.S. PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 U.S. NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 77 U.S. RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 CANADA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 CANADA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 CANADA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 CANADA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 CANADA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 CANADA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 CANADA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 85 CANADA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 86 CANADA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 CANADA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 CANADA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 CANADA NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 90 CANADA NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 CANADA NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 92 CANADA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 CANADA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 CANADA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 CANADA NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 96 CANADA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 MEXICO POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 MEXICO UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 MEXICO BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 MEXICO FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 MEXICO UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 MEXICO NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 MEXICO STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 104 MEXICO MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 105 MEXICO VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 MEXICO OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 MEXICO COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 MEXICO NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 109 MEXICO NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 MEXICO NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 111 MEXICO HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 MEXICO PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 MEXICO PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 MEXICO NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 115 MEXICO RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
图片列表
FIGURE 1 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE IS EXPECTED TO DRIVE THE NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 UTERINE BALLOON TAMPONADE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 15 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE (2024)
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
FIGURE 17 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2024
FIGURE 18 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 20 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 21 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2024
FIGURE 22 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 23 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, CAGR (2025-2032)
FIGURE 24 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 25 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2024
FIGURE 26 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2025-2032 (USD THOUSAND)
FIGURE 27 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, CAGR (2025-2032)
FIGURE 28 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, LIFELINE CURVE
FIGURE 29 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 30 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 31 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 32 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 33 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2024
FIGURE 34 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SNAPSHOT (2024)
FIGURE 38 NORTH AMERICA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY SHARE 2024 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。