Switzerland France Italy And Germany Cpap Devices Market
市场规模(十亿美元)
CAGR :
% 
 USD
874.25 Million
USD
1,348.35 Million
2025
2033
USD
874.25 Million
USD
1,348.35 Million
2025
2033
| 2026 –2033 | |
| USD 874.25 Million | |
| USD 1,348.35 Million | |
|
|
|
|
瑞士、法國、義大利及德國CPAP設備市場,依產品類型(CPAP設備、耗材)、應用領域(睡眠呼吸中止症、慢性阻塞性肺病(COPD)、肥胖低通氣症候群(OHS)、氣喘、毛細支氣管炎、其他)、模式(獨立式、可攜式)、最終用戶(家庭護理、醫院、私人診所、其他)、分銷管道(直接招標、零售、其他)、國家(德國、法國、義大利和瑞士)劃分-產業趨勢及至2033年的預測
瑞士、法國、義大利和德國CPAP設備市場規模
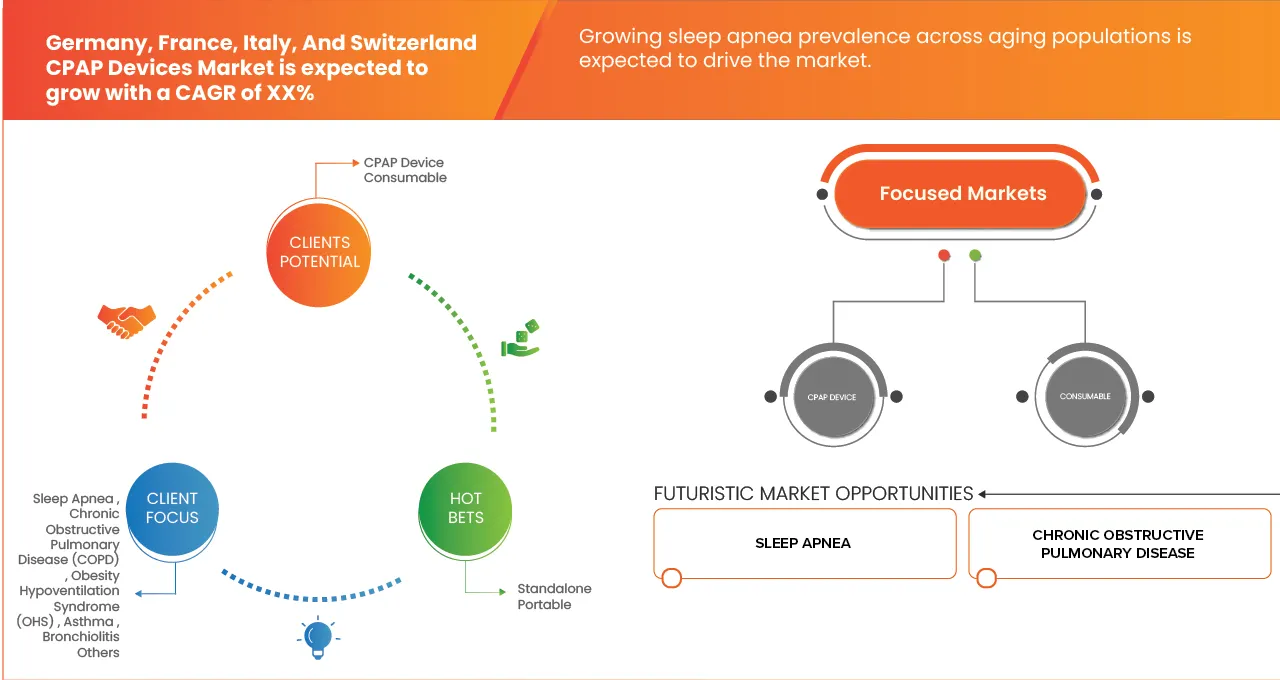
- 2025年,瑞士、法國、義大利和德國的CPAP設備市場價值為8.7425億美元,預計2033年將達到13.4835億美元。
- 在 2026 年至 2033 年的預測期內,市場可能會以5.6% 的複合年增長率增長,這主要得益於阻塞性睡眠呼吸中止症 (OSA) 患病率的上升、人們對睡眠相關疾病的認識不斷提高,以及睡眠實驗室和家庭睡眠測試的普及推動了診斷率的提高。
- 持續的市場成長得益於CPAP設備的技術進步,包括更安靜的馬達、更舒適的面罩、便攜易用的設計,以及遠端監測和雲端依從性追蹤等數位健康功能的整合。此外,醫保覆蓋範圍的擴大、家庭醫療保健的發展,以及透過醫院、睡眠診所和線上管道的強勁分銷,都在推動該地區CPAP設備的整體市場普及。
瑞士、法國、義大利和德國CPAP設備市場分析
- 隨著製造商日益注重技術創新、提升患者舒適度和優化治療方案,以滿足不斷變化的睡眠呼吸中止管理需求,瑞士、法國、義大利和德國的持續性正壓呼吸器(CPAP)設備市場正穩步發展。 CPAP設備開發商正利用更安靜的馬達技術、改進的壓力演算法、優化的面罩人體工學設計、加濕系統和便攜式設計,以提高患者在醫院、睡眠診所和家庭護理環境中的治療依從性。數位顯示器、基於雲端的依從性追蹤、行動應用程式連接和遠距醫療監測等功能的整合,正在進一步影響該地區的產品開發策略。
- 這些進步透過提高生產效率、產品可靠性和可擴展性,增強了區域CPAP價值鏈,同時支援長期成本優化。拓展直銷通路、經銷商網路以及與醫院和睡眠中心的合作,正在擴大市場滲透率並改善病患就醫途徑。在有利的報銷機制、政府睡眠健康計畫以及對慢性病管理日益重視的支持下,CPAP療法在家庭醫療保健環境中的普及率不斷提高,進一步提升了整體市場可及性。
- 預計到 2026 年,德國將以 42.28% 的最大收入份額主導瑞士、法國、義大利和德國 CPAP 設備市場,這得益於人們對睡眠障礙的高度認識、完善的睡眠醫學基礎設施以及製造商在產品創新、本地化生產和品牌擴張方面的持續投資。
- 法國預計將成為成長最快的市場,年複合成長率達 6.2%,這主要得益於阻塞性睡眠呼吸中止症的診斷率不斷提高、家庭呼吸照護的普及、遠距醫療整合的日益增長,以及透過醫院、藥房、線上平台和專業家庭護理提供者提高 CPAP 設備的可及性。
- 預計到2026年,CPAP設備細分市場將以62.52%的市佔率主導瑞士、法國、義大利和德國的CPAP設備市場,反映出市場對小巧、易用且經濟實惠的治療解決方案的強勁需求。 CPAP設備在醫院、睡眠診所和家庭護理機構的廣泛應用,以及在數位化監測和以患者為中心的設計方面的持續創新,將繼續鞏固該細分市場在該地區的領先地位。
報告範圍及瑞士、法國、義大利及德國CPAP設備市場細分
|
屬性 |
瑞士、法國、義大利和德國CPAP設備市場關鍵洞察 |
|
涵蓋部分 |
|
|
覆蓋國家/地區 |
|
|
主要市場參與者 |
|
|
市場機遇 |
|
|
加值資料資訊集 |
除了對市場狀況(如市場價值、成長率、細分、地理覆蓋範圍和主要參與者)的洞察之外,Data Bridge Market Research 精心編制的市場報告還包括深入的專家分析、患者流行病學、產品線分析、定價分析和監管框架。 |
瑞士、法國、義大利和德國CPAP設備市場趨勢
“拓展遠距醫療與遠距監測”
- 在瑞士、法國、義大利和德國,遠距醫療和遠端監測的擴展為CPAP設備帶來了巨大的發展機遇,徹底改變了睡眠呼吸中止症的管理和支援方式。隨著遠距醫療日益融入日常診療,患者無需頻繁到診即可接受睡眠呼吸障礙的診斷、處方和監測。這種便利性提高了醫療服務的可近性,尤其對於那些居住在偏遠或醫療資源匱乏地區的患者而言,他們原本可能會延誤就醫。現代CPAP設備內建的遠端監測功能使醫護人員能夠即時追蹤使用模式、治療效果和患者依從性,從而及時調整治療方案並提供個人化支援。這種互聯互通加強了醫病關係,並增強了患者對治療結果的信心。此外,遠距醫療平台還支援患者教育和故障排除,從而降低了患者有效使用CPAP設備的障礙。透過簡化醫療服務流程和提高長期依從性,不斷發展的遠距醫療生態系統推動了CPAP設備在這些歐洲醫療市場的普及和持續需求。
- 根據國際貿易管理局的數據,截至 2025 年 7 月,德國遠距醫療市場(不包括數位健身產品和健康追蹤器)預計將從 2024 年的 27.9 億美元成長到 2030 年的 44 億美元。
- 根據格萊斯·盧茨律師事務所2025年8月發表的文章,德國的遠距醫療基礎設施近年來取得了顯著的改進和擴展。先前對視訊諮詢比例的法律限制已被取消,這使得德國醫療行業在提供此類諮詢方面擁有更大的靈活性和範圍。
- 根據美國國家醫學圖書館2025年11月的數據顯示,法國衛生部在2009年至2022年間資助了41個遠距醫療研究項目,總額達1,747萬美元或1,750萬美元。
- 總體而言,瑞士、法國、義大利和德國遠距醫療和遠距監測的快速發展,透過改善病患的就醫途徑、依從性和臨床監管,促進了CPAP治療的普及。數位化連結實現了治療方案的持續優化和患者的積極參與,使CPAP成為更便捷、數據驅動且被廣泛接受的長期睡眠呼吸中止症管理方案。
瑞士、法國、義大利和德國CPAP設備市場動態
司機
“高額醫療保健支出”
- 瑞士、法國、義大利和德國等國的高醫療保健支出創造了有利的環境,顯著提升了對持續性正壓呼吸器(CPAP)設備的需求。這些國家將相當一部分國家預算用於醫療服務,這意味著完善的保險覆蓋、先進的醫療基礎設施以及對預防和慢性病管理的重視。在這樣的體系下,阻塞性睡眠呼吸中止症等睡眠障礙日益被視為嚴重的健康問題,而非小眾疾病。高醫療保健支出通常與更廣泛的宣傳活動、更完善的診斷設施和更頻繁的篩檢有關,所有這些都提高了睡眠呼吸障礙的檢出率。
- 在這些國家,一旦確診,患者更有可能獲得補貼或報銷的醫療器械,從而降低了可能阻礙他們購買CPAP呼吸機的經濟障礙。此外,資金充足的醫療保健系統往往會採用並推廣高品質的醫療技術,鼓勵醫療服務提供者將CPAP療法作為睡眠呼吸中止症的第一線治療方案。這不僅增強了臨床醫生推薦這些設備的信心,也讓患者對其有效性和安全性更加放心。強大的公共和私人醫療保險體系、高人均醫療支出以及對健康和長期健康結果的重視,共同推動了對CPAP設備的持續需求。本質上,高額的醫療保健支出不僅改善了醫療服務的可近性,還影響著從診斷到治療依從性的整個醫療服務鏈,從而提升了這些歐洲市場對CPAP療法的需求。
- 2025 年 7 月,Feather Insurance 發表了一篇部落格文章,指出德國在歐洲醫療保健領域投入的 GDP 比例最高,達到 12.8%。
- 根據歐盟統計局的數據,截至 2025 年 10 月,在歐盟國家中,德國(11.7%)、法國(11.5%)、奧地利和瑞典(均為 11.2%)2023 年的醫療保健支出佔 GDP 的比例最高。
- 總體而言,瑞士、法國、義大利和德國持續高企的醫療保健支出為CPAP的廣泛應用創造了有利的財政和結構性支持環境。強大的保險覆蓋、政府支持的報銷體係以及先進的醫院和睡眠護理基礎設施確保了患者在確診後能夠及時獲得治療。隨著睡眠呼吸中止症日益被視為一種嚴重的慢性疾病而非生活方式疾病,資金充足的醫療保健系統使醫生能夠自信地開立CPAP處方,也使患者能夠堅持長期治療。這降低了患者對價格的敏感性,加速了技術的普及,並維持了對CPAP設備及配件的持續需求,從而鞏固了這些成熟的歐洲醫療保健體系中CPAP市場的穩定成長。
限制/挑戰
“設備和配件成本高昂”
- 在瑞士、法國、義大利和德國,高昂的設備及配件成本嚴重阻礙了CPAP設備的需求,造成經濟障礙,令病患和醫療服務提供者望而卻步。即使在醫療體系相對完善的國家,昂貴的CPAP設備以及面罩、管路和過濾器等配件的持續費用也會使人們不願接受治療,尤其是在保險覆蓋範圍有限或報銷流程複雜的情況下。高昂的前期費用可能導致患者延遲或放棄購買CPAP設備,特別是那些沒有症狀或認為睡眠問題並不緊急的患者。
- 此外,由於擔心患者無法負擔必要的更換和升級費用,醫療服務提供者在建議長期治療時可能會遇到阻力。這會導致依從率降低和整體市場滲透率下降。最終,當購置和維護CPAP設備的經濟負擔超過了其帶來的益處時,需求就會減弱,即使在醫療資源豐富的市場,市場成長也會放緩。
- 根據睡眠醫師控股有限公司(Sleep Doctor Holdings, LLC)預測,到2025年12月,一台持續性正壓呼吸器(CPAP)機的價格可能在500美元到1000美元甚至更高,功能越先進的CPAP機價格通常越高。預計這將增加患者的自付費用,尤其是那些沒有全額保險的患者,從而抑制市場需求。
- 根據 Sleep Doctor Holdings, LLC 的數據,截至 2025 年 12 月,CPAP 呼吸器空氣過濾器的價格通常為每個 5 美元或更低,具體價格取決於機器類型。空氣濾網應每月更換一次。標準呼吸管的價格可能在 5 美元到 35 美元之間,而加熱型呼吸管的價格更高,在 30 美元到 75 美元之間。呼吸管應每三個月更換一次。頭帶和麵罩的價格通常在 50 美元到 200 美元之間,具體價格取決於設計。這些部件應每六個月更換一次。預計持續的成本將阻礙市場成長。
- 總體而言,高昂的設備和配件成本仍然是瑞士、法國、義大利和德國等醫療資源充足的國家推廣CPAP治療的一大障礙。前期投入龐大,加上面罩、管路和過濾器頻繁更換,增加了治療的終身成本,導致許多患者猶豫是否開始或繼續治療。如果報銷不全或手續繁瑣,自付費用會進一步降低患者的長期堅持治療的意願。這種經濟負擔也使得臨床醫師在為輕症或早期患者開立CPAP處方時更加謹慎。因此,儘管臨床需求強烈,但價格壓力限制了市場滲透率,減緩了CPAP設備在這些成熟的歐洲市場的普及速度。
瑞士、法國、義大利和德國CPAP設備市場範圍
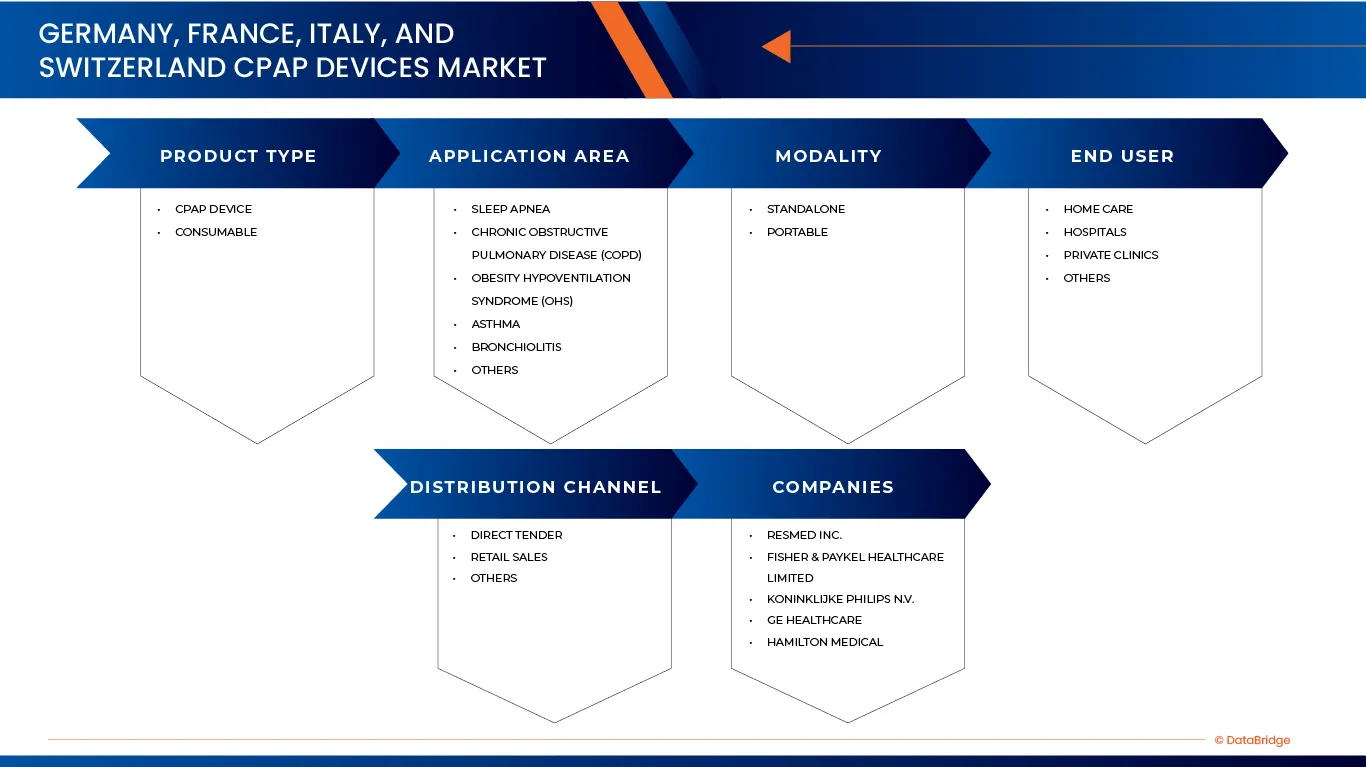
德國、法國、義大利和瑞士的 CPAP 設備市場根據產品類型、應用領域、模式、最終用戶和分銷管道分為五個主要部分。
- 依產品類型
根據產品類型,德國、法國、義大利和瑞士的CPAP設備市場分為CPAP設備和耗材兩大類。預計到2026年,CPAP設備將佔據市場主導地位,市佔率高達62.52%,主要得益於醫院、睡眠診所、社區醫療中心和家庭護理機構的廣泛應用。老舊設備的強勁更換需求、阻塞性睡眠呼吸中止症診斷率的不斷提高,以及消費者對便攜式、小巧便捷的CPAP系統的日益青睞,都鞏固了該細分市場的領先地位。壓力輸送演算法、降噪、加濕和數位化依從性監測等方面的持續創新,也進一步支撐了市場的持續需求。
在瑞士、法國、義大利和德國的持續性正壓呼吸器(CPAP)設備市場中,耗材細分市場成長最快,複合年增長率(CAGR)達5.9%。這主要得益於對面罩、管路、過濾器、頭帶和加濕器等耗材的持續需求,以及患者長期治療依從性的提高和居家睡眠呼吸中止症管理模式的日益普及。此外,直銷通路、線上藥局、訂閱式更換模式和零售分銷網路的擴張,以及面罩舒適度、耐用性和材料品質的不斷提升,預計將進一步加速該地區CPAP設備的普及。
- 按應用領域
根據應用領域,德國、法國、義大利和瑞士的CPAP設備市場可細分為睡眠呼吸中止症、慢性阻塞性肺病(COPD)、肥胖低通氣症候群(OHS)、氣喘、毛細支氣管炎和其他疾病。預計到2026年,睡眠呼吸中止症細分市場將佔據主導地位,市場份額高達84.52%,這主要得益於阻塞性睡眠呼吸中止症(OSA)的高發病率以及CPAP療法作為一線治療手段的廣泛應用。醫院、睡眠診所、專業呼吸照護中心和家庭護理機構的廣泛採用,以及CPAP療法已被證實的臨床療效、長期治療需求和完善的醫療覆蓋,都將繼續鞏固該細分市場的領先地位。
在瑞士、法國、義大利和德國的CPAP設備市場中,睡眠呼吸中止症細分市場是成長最快的細分市場,複合年增長率達5.7%。這主要得益於睡眠呼吸障礙診斷率的上升、人們對未經治療的阻塞性睡眠呼吸中止症(OSA)所帶來的長期心血管和代謝風險的認識不斷提高,以及消費者越來越傾向於選擇便攜式、易用且具備數位化功能的CPAP設備進行居家治療。遠端監測、基於雲端的依從性追蹤和遠距醫療支援的睡眠照護的整合,以及透過家庭醫療保健服務提供者和線上分銷管道擴大服務範圍,預計將進一步加速這些設備在主要區域市場的普及。
- 按模式
根據使用模式,德國、法國、義大利和瑞士的CPAP設備市場分為獨立式和可攜式兩大類。預計到2026年,獨立式CPAP設備將佔據市場主導地位,市佔率高達86.09%,這主要得益於其在臨床和家庭護理環境中廣泛用於長期、夜間睡眠呼吸中止症治療。獨立式CPAP設備提供穩定的壓力輸出、先進的加濕功能、更舒適的佩戴體驗和更高的耐用性,使其非常適合連續使用。此外,其相對較低的單次治療成本、易於維護以及在醫院、睡眠診所和家庭護理環境中的廣泛應用,也鞏固了其市場領先地位。
在瑞士、法國、義大利和德國的CPAP設備市場中,便攜式CPAP設備是成長最快的細分市場,複合年增長率達6.0%。這主要得益於消費者對輕便、便於攜帶和緊湊型CPAP解決方案的需求不斷增長,尤其是在職場人士、經常出差旅行的人以及需要在戶外持續接受治療的患者群體中。此外,消費者對電池供電和USB相容設備的偏好日益增強,以及行動連接、遠端依從性追蹤和遠距醫療支援的睡眠護理等功能的集成,也進一步加速了便攜式CPAP設備在主要區域市場的普及。
- 最終用戶
根據最終用戶,德國、法國、義大利和瑞士的CPAP設備市場可細分為家庭護理、醫院、私人診所和其他。預計到2026年,家庭護理領域將以65.52%的市場份額佔據主導地位,這主要得益於睡眠呼吸中止症和其他慢性呼吸系統疾病患者越來越多地選擇居家治療。在居家照護環境下的CPAP治療為患者提供了更大的便利性,提高了治療依從性,並具有長期成本效益,從而促進了其廣泛應用。使用者友善、數位化CPAP設備的日益普及,以及強有力的報銷支持和不斷擴展的家庭醫療保健服務,持續鞏固了該領域的領先地位。
在瑞士、法國、義大利和德國,家庭護理是CPAP設備市場中成長最快的細分領域,複合年增長率達5.9%。這主要得益於阻塞性睡眠呼吸中止症診斷率的上升、人口老化以及消費者對非住院、持續治療方案的日益青睞。遠端患者監測、基於雲端的依從性追蹤和遠距醫療支援的睡眠護理技術的進步,以及直接面向消費者的銷售、電子藥房和家庭護理服務提供者網路的擴張,正在進一步加速CPAP設備在主要區域市場的普及。
- 透過分銷管道
根據分銷管道,德國、法國、義大利和瑞士的CPAP設備市場可分為直接招標、零售和其他通路。預計到2026年,直接招標管道將佔據市場主導地位,市場份額高達59.56%,這主要得益於公立醫院、睡眠診所和政府支持的醫療機構的大批量採購。直接招標機制能夠有效降低成本、實現設備標準化部署並簽訂長期供應合同,使其成為機構採購CPAP設備的首選管道。製造商、醫療機構和集團採購組織之間的牢固合作關係,以及包含設備、耗材和服務合約的捆綁式產品,都進一步鞏固了該管道的領先地位。
在瑞士、法國、義大利和德國,零售額是CPAP設備市場成長最快的細分領域,複合年增長率達5.9%。這主要得益於居家睡眠呼吸中止症管理模式的日益普及、病患意識的提高以及CPAP設備在藥局、專業醫療器材商店、電商平台和家庭護理服務提供者等管道的廣泛普及。此外,直銷模式的擴展、報銷政策的完善以及便捷易用的便攜式CPAP系統的推出,也進一步加速了CPAP設備在主要區域市場的普及。
瑞士、法國、義大利和德國CPAP設備市場區域分析
- 預計到 2026 年,德國將以 42.28% 的最大收入份額主導 CPAP 設備市場,這得益於德國對醫療保健基礎設施的大量投資、強大的國內診斷製造能力以及完善的即時檢測生態系統。
- 人們對預防性醫療保健的高度重視、對快速可靠診斷的需求不斷增長,以及醫院、診所、實驗室、藥房和家庭護理管道中快速診斷試劑的廣泛普及,持續鞏固了德國在區域市場的領先地位。
法國CPAP設備市場洞察
法國CPAP設備市場正穩定成長,這主要得益於人們對睡眠障礙的認識不斷提高、阻塞性睡眠呼吸中止症(OSA)診斷率上升以及完善的醫療保健和報銷體系。居家睡眠呼吸中止症管理模式的日益普及,以及有利的保險覆蓋和強大的家庭醫療保健網絡,顯著改善了患者的就醫途徑和長期治療依從性。醫院、睡眠診所、藥房、專業家庭護理機構和線上管道的廣泛銷售,進一步促進了CPAP設備的普及。此外,數位化監測、遠距醫療支援的睡眠照護以及連網CPAP平台的日益融合,也持續鞏固了法國在歐洲CPAP設備市場的地位。
義大利CPAP設備市場洞察
義大利CPAP設備市場預計將穩定成長,這得益於人們健康意識的提高、與老化和肥胖相關的睡眠呼吸中止症盛行率的上升,以及診斷和呼吸護理服務的逐步改善。家庭CPAP療法的日益普及,以及私人睡眠診所和呼吸照護機構的擴張,都促進了市場成長。電子藥局平台、直銷模式和家庭護理服務提供者的日益增多,提高了設備的可及性,尤其是在城市和半城市地區。此外,消費者對價格合理、可靠且易於使用的CPAP系統的需求不斷增長,加上設備舒適度、便攜性和數位化依從性追蹤功能的不斷進步,共同推動了義大利市場的持續發展。
市場上的主要市場領導者包括:
- 瑞思邁公司(美國)
- Fisher & Paykel Healthcare Limited(紐西蘭)
- Koninklijke Philips NV(荷蘭)
- GE醫療(美國)
- 漢密爾頓醫療(瑞士)
- Nareena Lifesciences Private Limited(印度)
- BMC醫療股份有限公司(中國)
- Wellell Inc.(台灣)
- Fritz Stephan GmbH(德國)
- 維貢(法國)
- Löwenstein Medical SE & Co. KG(德國)
- SOMNOmedics AG(德國)
- CVR醫療用品(德國)
- Kranz GmbH(德國)
- ECUMED GmbH(德國)
- Boppel.Med(德國)
- 納朗醫療有限公司(印度)
- 海爾醫療股份公司(德國)
- 湖南博遠醫療科技股份有限公司(中國)
- 日本光電株式會社(日本)
- 魏因曼急救醫療技術(德國)
- 塞法姆(法國)
瑞士、法國、義大利和德國CPAP設備的最新發展
- 2025 年 9 月,SOMNOmedics AG 與 Cognision 建立了策略合作夥伴關係,將 Cognision 先進的多導睡眠圖 (PSG) 系統整合到製藥公司贊助的臨床試驗中,從而擴大了其睡眠結構測量技術在受監管的研究環境中的應用。
- 2024年7月,WEINMANN Emergency發布了MEDUCORE Standard²監視器/除顫器3.9版本軟體更新,引入了一系列增強功能,旨在提升易用性、效能和連接性。此更新使用戶能夠直接在設備上配置Wi-Fi設置,新增了對印尼語和羅馬尼亞語的支持,並支援透過WEINMANN Connect進行集中同步,實現自動夏令時調整。其他升級包括更快的會話存檔存取速度、改進的AED退出模式使用者指南、自動檢測SpO₂電纜問題以及自動上傳新註冊設備的服務數據,所有這些改進都旨在簡化操作並支援可靠的急救護理。
- 2022年3月,專業另一個性醫療器材集團Vygon宣布收購位於秘魯利馬的經銷商Macatt Medica。該公司在秘魯經銷Vygon的大部分產品,包括各種腸內營養產品。
- 2022年9月,Sefam Medical推出了由Starck設計的Sefam S.Box,為睡眠呼吸中止症治療帶來了一種全新的方法。該產品將先進的CPAP療法與數位連接和以患者為中心的設計相結合。該平台整合了無線連接功能,並為患者和臨床醫生配備了配套應用程序,能夠即時監測治療依從性、睡眠品質和治療效果。透過支援遠端追蹤、個人化護理和提高患者參與度,S.Box旨在提升治療效果,同時減輕醫療負擔並降低整體治療成本。
- 2025年12月,瑞思邁(ResMed)的個人化治療舒適設定(PTCS)獲得美國FDA核准,並將以「智慧舒適」(Smart Comfort)為名上市。這是首款由人工智慧驅動的數位醫療設備,旨在為持續性正壓呼吸器(CPAP)治療推薦個人化的舒適設置,幫助阻塞性睡眠呼吸中止症患者開始並堅持治療。智慧舒適利用基於超過1億個真實睡眠夜晚資料訓練的機器學習演算法,透過myAir應用程式為AirSense 11裝置的用戶量身打造壓力上升和呼氣釋放等設定。該產品將於2026年初在美國分階段上市。此次獲準進一步鞏固了瑞思邁專注於數據驅動的個人化睡眠健康解決方案,旨在提升患者的舒適度和治療依從性。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 DBMR VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.1.1 POLITICAL FACTORS
4.1.2 ECONOMIC FACTORS
4.1.3 SOCIAL FACTORS
4.1.4 TECHNOLOGICAL FACTORS
4.1.5 LEGAL FACTORS
4.1.6 ENVIRONMENTAL FACTORS
4.2 OPPORTUNITY MAP ANALYSIS
4.2.1 INTRODUCTION
4.2.2 HEALTHCARE EXPENDITURE
4.2.3 CAPITAL EXPENDITURE
4.2.4 CAPEX TRENDS
4.2.5 CAPEX ALLOCATION
4.2.6 FUNDING SOURCES
4.2.7 INDUSTRY BENCHMARKS
4.2.8 HEALTHCARE SPENDING AS RATIO OF OVERALL GDP
4.2.9 HEALTHCARE SYSTEM STRUCTURE
4.2.10 GOVERNMENT POLICIES
4.2.11 ECONOMIC DEVELOPMENT
4.3 INDUSTRY INSIGHTS
4.3.1 MICRO AND MACRO ECONOMIC FACTORS
4.3.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.3.3 KEY PRICING STRATEGIES
4.3.4 INTERVIEWS WITH SPECIALISTS
4.3.5 ANALYSIS AND RECOMMENDATIONS
4.4 COST ANALYSIS BREAKDOWN
4.4.1 INTRODUCTION
4.4.2 MARKET COST STRUCTURE OVERVIEW
4.4.3 DEVICE ACQUISITION COST ANALYSIS
4.4.4 MASK AND CONSUMABLES COST DYNAMICS
4.4.5 SERVICE AND FOLLOW-UP COST ASSESSMENT
4.4.6 REIMBURSEMENT IMPACT ON NET COST
4.4.7 TOTAL COST OF OWNERSHIP (TCO) ANALYSIS
4.4.8 STRATEGIC MARKET IMPLICATIONS
4.4.9 CONCLUSION
4.5 OPPORTUNITY MAP ANALYSIS
4.5.1 INTRODUCTION
4.5.2 OPPORTUNITY LANDSCAPE ACROSS DISEASE AWARENESS AND DIAGNOSIS PATHWAYS
4.5.3 OPPORTUNITY MAPPING BY THERAPY ADHERENCE AND LONG-TERM COMPLIANCE GAPS
4.5.4 OPPORTUNITY MAPPING BY HOMECARE AND DECENTRALIZED CARE EXPANSION
4.5.5 OPPORTUNITY MAPPING BY TECHNOLOGY DIFFERENTIATION AND DIGITAL INTEGRATION
4.5.6 OPPORTUNITY MAPPING BY REIMBURSEMENT EVOLUTION AND VALUE-BASED CARE
4.5.7 CONCLUSION
4.6 REIMBURSEMENT FRAMEWORK FOR CPAP DEVICES
4.6.1 INTRODUCTION
4.6.2 ELIGIBILITY CRITERIA AND DIAGNOSTIC PATHWAYS
4.6.3 DEVICE COVERAGE STRUCTURE AND COST COMPONENTS
4.6.4 ROLE OF HEALTHCARE PROVIDERS AND DISTRIBUTION CHANNELS
4.6.5 ADHERENCE MONITORING AND CONDITIONAL REIMBURSEMENT
4.6.6 PATIENT COST SHARING AND FINANCIAL RESPONSIBILITY
4.6.7 CONCLUSION
4.7 SUPPLY CHAIN ECOSYSTEM
4.7.1 PROMINENT COMPANIES
4.7.2 SMALL & MEDIUM SIZE COMPANIES
4.7.3 END USERS
4.8 TECHNOLOGY ROADMAP
4.8.1 INTRODUCTION
4.8.2 SHORT-TERM ROADMAP (2024–2026): CONNECTIVITY, COMFORT, AND COMPLIANCE
4.8.2.1 CORE TECHNOLOGY TRENDS
4.8.2.2 DIGITAL CONNECTIVITY INTEGRATION
4.8.2.3 COUNTRY DIFFERENTIATION
4.8.3 MID-TERM ROADMAP (2026–2029): AI-DRIVEN THERAPY OPTIMIZATION AND ECOSYSTEM INTEGRATION
4.8.3.1 ARTIFICIAL INTELLIGENCE AND PREDICTIVE ANALYTICS
4.8.3.2 INTEGRATION WITH DIGITAL HEALTH ECOSYSTEMS
4.8.3.3 REMOTE CARE AND HOMECARE AUTOMATION
4.8.3.4 COUNTRY DIFFERENTIATION
4.8.4 LONG-TERM ROADMAP (2029–2035): PREVENTIVE SLEEP MEDICINE AND THERAPY CONVERGENCE
4.8.4.1 THERAPY CONVERGENCE AND HYBRID DEVICES
4.8.4.2 PREDICTIVE POPULATION HEALTH APPLICATIONS
4.8.4.3 MINIATURIZATION AND LIFESTYLE INTEGRATION
4.8.5 REGULATORY AND REIMBURSEMENT INFLUENCE ON THE ROADMAP
4.8.6 STRATEGIC IMPLICATIONS FOR MANUFACTURERS
4.8.7 CONCLUSION
4.9 COMPANY EVALUATION QUADRANT
4.1 INNOVATION TRACKER AND STRATEGIC ANALYSIS
4.10.1 INTRODUCTION
4.10.2 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
4.10.2.1 JOINT VENTURES
4.10.2.2 MERGERS AND ACQUISITIONS
4.10.2.3 LICENSING AND PARTNERSHIPS
4.10.2.4 TECHNOLOGY COLLABORATIONS
4.10.2.5 STRATEGIC DIVESTMENTS
4.10.3 NUMBER OF PRODUCTS IN DEVELOPMENT
4.10.4 STAGE OF DEVELOPMENT
4.10.5 TIMELINES AND MILESTONES
4.10.6 INNOVATION STRATEGIES AND METHODOLOGIES
4.10.7 RISK ASSESSMENT AND MITIGATION
4.10.8 FUTURE OUTLOOK
5 SALES VOLUME PER PLAYER IN THE SWITZERLAND, FRANCE, ITALY AND GERMANY (UNIT)
6 REGULATORY COMPLIANCE ANALYSIS
6.1 INTRODUCTION
6.2 REGULATORY AUTHORITIES
6.3 REGULATORY CLASSIFICATIONS
6.4 REGULATORY SUBMISSIONS
6.5 CONCLUSION
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING PREVALENCE OF SLEEP APNEA AND COPD
7.1.2 HIGH HEALTHCARE SPENDING
7.1.3 RISING AGING DEMOGRAPHICS
7.2 RESTRAINTS
7.2.1 HIGH DEVICE & ACCESSORY COSTS
7.2.2 REGULATORY COMPLEXITY & APPROVAL DELAYS
7.3 OPPORTUNITIES
7.3.1 EXPANDING TELEMEDICINE & REMOTE MONITORING
7.3.2 GROWING HOMECARE & OUTPATIENT CLINICS NETWORK
7.3.3 TECHNOLOGICAL ADVANCEMENTS IN CPAP DEVICES
7.4 CHALLENGES
7.4.1 SUPPLY CHAIN & COMPONENT SHORTAGES
7.4.2 PATIENT NON-ADHERENCE
8 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 CPAP DEVICE
8.2.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
8.2.1.1 AUTO-ADJUSTING CPAP DEVICES
8.2.1.1.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICE IN CPAP DEVICES MARKET, BY AUTOMATION, 2018-2033 (USD THOUSAND)
8.2.1.1.1.1 AUTOMATIC
8.2.1.1.1.2 MANUAL
8.2.1.2 FIXED PRESSURE CPAP DEVICES
8.2.1.3 TRAVEL CPAP DEVICES
8.3 CONSUMABLE
8.3.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND CONSUMABLE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
8.3.1.1 CPAP MASK
8.3.1.1.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP MASK IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
8.3.1.1.1.1 NASAL MASKS
8.3.1.1.1.2 FULL-FACE MASKS
8.3.1.1.1.3 NASAL PILLOW MASKS
8.3.1.2 CPAP HOSES
8.3.1.3 HUMIDIFIER WATER CHAMBERS
8.3.1.4 CPAP FILTERS
8.3.1.4.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP FILTERS IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
8.3.1.4.1.1 DISPOSABLE FILTERS
8.3.1.4.1.2 REUSABLE FILTERS
8.3.1.5 CPAP MOTORS
8.3.1.6 CHIN STRAPS
8.3.1.7 OTHERS
9 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY APPLICATION AREA
9.1 OVERVIEW
9.2 SLEEP APNEA
9.2.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND SLEEP APNEA IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
9.2.1.1 OBSTRUCTIVE SLEEP APNEA
9.2.1.2 CENTRAL SLEEP APENA
9.2.1.3 MIXED SLEEP APENA
9.3 CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
9.4 OBESITY HYPOVENTILATION SYNDROME (OHS)
9.5 ASTHMA
9.6 BRONCHIOLITIS
9.7 OTHERS
10 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY MODALITY
10.1 OVERVIEW
10.2 STANDALONE
10.3 PORTABLE
11 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOME CARE
11.2.1 SWITZERLAND, FRANCE, ITALY, AND GERMANY HOME CARE IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
11.2.1.1 STANDALONE
11.2.1.2 PORTABLE
11.3 HOSPITALS
11.3.1 SWITZERLAND, FRANCE, ITALY, AND GERMANY HOSPITALS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
11.3.1.1 STANDALONE
11.3.1.2 PORTABLE
11.4 PRIVATE CLINICS
11.4.1 SWITZERLAND, FRANCE, ITALY, AND GERMANY PRIVATE CLINICS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
11.4.1.1 STANDALONE
11.4.1.2 PORTABLE
11.5 OTHERS
11.5.1 SWITZERLAND, FRANCE, ITALY, AND GERMANY OTHERS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
11.5.1.1 STANDALONE
11.5.1.2 PORTABLE
12 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
12.3.1 GERMANY, FRANCE, ITALY, AND SWITZERLAND RETAIL SALES IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
12.3.1.1 OFFLINE
12.3.1.2 ONLINE
12.4 OTHERS
13 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET, BY COUNTRY
13.1 OVERVIEW
13.2 GERMANY
13.3 FRANCE
13.4 ITALY
13.5 SWITZERLAND
14 GERMANY, SWITZERLAND, FRANCE, ITALY CPAP DEVICES MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GERMANY, SWITZERLAND, FRANCE, ITALY
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 RESMED
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT UPDATES
16.2 FISHER & PAYKEL HEALTHCARE LIMITED
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENT
16.3 KONINKLIJKE PHILIPS N.V.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 GE HEALTHCARE
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 WELLELL INC.
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENT
16.6 BMC MEDICAL CO., LTD
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 BOPPEL.MED
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 CVR MEDICAL SUPPLY.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ECUMED GMBH.
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 FRITZ STEPHAN GMBH
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 HAMILTON MEDICAL
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 HEYER MEDICAL AG
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 HUNAN BEYOND MEDICAL TECHNOLOGY CO., LTD.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 KRANZ GMBH.
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENT
16.15 LÖWENSTEIN MEDICAL SE & CO. KG.
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
16.16 NARANG MEDICAL LIMITED
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 NAREENA LIFESCIENCES PVT.LIMITED
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT
16.18 NIHON KOHDEN CORPORATION
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT UPDATES
16.19 SEFAM
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT UPDATES
16.2 SOMNOMEDICS AG.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENT
16.21 VYGON
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
表格列表
TABLE 1 STRATEGIC OPPORTUNITY HEAT MAP FOR CPAP DEVICES MARKET IN SWITZERLAND, FRANCE, ITALY, AND GERMANY
TABLE 2 COUNTRY-SPECIFIC STRATEGIC OPPORTUNITY MATRIX FOR CPAP DEVICES MARKET
TABLE 3 COUNTRY-WISE REIMBURSEMENT COMPARISON (CPAP DEVICES)
TABLE 4 REGULATORY–REIMBURSEMENT CROSS-IMPACT ANALYSIS
TABLE 5 PAYER-CENTRIC RISK AND OPPORTUNITY MATRIX
TABLE 6 COUNTRY-WISE REGULATORY REQUIREMENTS FOR CPAP DEVICES
TABLE 7 REGULATORY RISK HEAT MAP FOR CPAP MANUFACTURERS
TABLE 8 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 9 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 10 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICE IN CPAP DEVICES MARKET, BY AUTOMATION, 2018-2033 (USD THOUSAND)
TABLE 11 GERMANY, FRANCE, ITALY, AND SWITZERLAND CONSUMABLE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 12 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP MASK IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 13 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP FILTERS IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 14 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 15 GERMANY, FRANCE, ITALY, AND SWITZERLAND SLEEP APNEA IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 16 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 17 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 18 SWITZERLAND, FRANCE, ITALY, AND GERMANY HOME CARE IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 19 SWITZERLAND, FRANCE, ITALY, AND GERMANY HOSPITALS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 20 SWITZERLAND, FRANCE, ITALY, AND GERMANY PRIVATE CLINICS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 21 SWITZERLAND, FRANCE, ITALY, AND GERMANY OTHERS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 22 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 23 GERMANY, FRANCE, ITALY, AND SWITZERLAND RETAIL SALES IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 24 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET, BY COUNTRY, 2018-2033 (USD THOUSAND)
TABLE 25 GERMANY CPAP DEVICES MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 26 GERMANY CPAP DEVICE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 27 GERMANY CPAP DEVICE IN CPAP DEVICES MARKET, BY AUTOMATION, 2018-2033 (USD THOUSAND)
TABLE 28 GERMANY CONSUMABLE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 29 GERMANY CPAP MASK IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 30 GERMANY CPAP FILTERS IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 31 GERMANY CPAP DEVICES MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 32 GERMANY SLEEP APNEA IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 33 GERMANY CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 34 GERMANY CPAP DEVICES MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 35 GERMANY HOME CARE IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 36 GERMANY HOSPITALS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 37 GERMANY PRIVATE CLINICS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 38 GERMANY OTHERS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 39 GERMANY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL , 2018-2033 (USD THOUSAND)
TABLE 40 GERMANY RETAIL SALES IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 41 FRANCE CPAP DEVICES MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 42 FRANCE CPAP DEVICE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 43 FRANCE CPAP DEVICE IN CPAP DEVICES MARKET, BY AUTOMATION, 2018-2033 (USD THOUSAND)
TABLE 44 FRANCE CONSUMABLE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 45 FRANCE CPAP MASK IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 46 FRANCE CPAP FILTERS IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 47 FRANCE CPAP DEVICES MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 48 FRANCE SLEEP APNEA IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 49 FRANCE CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 50 FRANCE CPAP DEVICES MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 51 FRANCE HOME CARE IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 52 FRANCE HOSPITALS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 53 FRANCE PRIVATE CLINICS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 54 FRANCE OTHERS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 55 FRANCE CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL , 2018-2033 (USD THOUSAND)
TABLE 56 FRANCE RETAIL SALES IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 57 ITALY CPAP DEVICES MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 58 ITALY CPAP DEVICE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 59 ITALY CPAP DEVICE IN CPAP DEVICES MARKET, BY AUTOMATION, 2018-2033 (USD THOUSAND)
TABLE 60 ITALY CONSUMABLE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 61 ITALY CPAP MASK IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 62 ITALY CPAP FILTERS IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 63 ITALY CPAP DEVICES MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 64 ITALY SLEEP APNEA IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 65 ITALY CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 66 ITALY CPAP DEVICES MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 67 ITALY HOME CARE IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 68 ITALY HOSPITALS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 69 ITALY PRIVATE CLINICS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 70 ITALY OTHERS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 71 ITALY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL , 2018-2033 (USD THOUSAND)
TABLE 72 ITALY RETAIL SALES IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 73 SWITZERLAND CPAP DEVICES MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 74 SWITZERLAND CPAP DEVICE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 75 SWITZERLAND CPAP DEVICE IN CPAP DEVICES MARKET, BY AUTOMATION, 2018-2033 (USD THOUSAND)
TABLE 76 SWITZERLAND CONSUMABLE IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 77 SWITZERLAND CPAP MASK IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 78 SWITZERLAND CPAP FILTERS IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 79 SWITZERLAND CPAP DEVICES MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 80 SWITZERLAND SLEEP APNEA IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 81 SWITZERLAND CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 82 SWITZERLAND CPAP DEVICES MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 83 SWITZERLAND HOME CARE IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 84 SWITZERLAND HOSPITALS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 85 SWITZERLAND PRIVATE CLINICS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 86 SWITZERLAND OTHERS IN CPAP DEVICES MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 87 SWITZERLAND CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL , 2018-2033 (USD THOUSAND)
TABLE 88 SWITZERLAND RETAIL SALES IN CPAP DEVICES MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
图片列表
FIGURE 1 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: SEGMENTATION
FIGURE 2 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: DROC ANALYSIS
FIGURE 4 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: GLOBAL VS REGIONAL ANALYSIS
FIGURE 5 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 STRATEGIC DECISIONS
FIGURE 12 TWO SEGMENT COMPRISE THE GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET, BY PRODUCT TYPE (2025)
FIGURE 13 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: SEGMENTATION
FIGURE 14 RISING PREVALENCE OF SLEEP APNEA AND COPD EXPECTED TO DRIVE THE GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET IN THE FORECAST PERIOD OF 2026 TO 2033
FIGURE 15 CPAP DEVICES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET IN 2026 & 2033
FIGURE 16 DRIVER, RESTRAINTS, OPPORTUNITIES, CHALLENGES ANALYSIS OF SWITZERLAND, FRANCE, ITALY AND GERMANY CPAP DEVICES MARKET
FIGURE 17 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY PRODUCT TYPE, 2025
FIGURE 18 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET: BY PRODUCT TYPE, 2026-2033 (USD THOUSAND)
FIGURE 19 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET: BY PRODUCT TYPE, CAGR (2026-2033)
FIGURE 20 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 21 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY APPLICATION AREA, 2025
FIGURE 22 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET: BY APPLICATION AREA, 2026-2033 (USD THOUSAND)
FIGURE 23 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET: BY APPLICATION AREA, CAGR (2026-2033)
FIGURE 24 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET: BY APPLICATION AREA, LIFELINE CURVE
FIGURE 25 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY MODALITY, 2025
FIGURE 26 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY MODALITY, 2026-2033 (USD THOUSAND)
FIGURE 27 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY MODALITY, CAGR (2026-2033)
FIGURE 28 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY MODALITY, LIFELINE CURVE
FIGURE 29 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY END USER, 2025
FIGURE 30 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY END USER, 2026-2033 (USD THOUSAND)
FIGURE 31 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY END USER, CAGR (2026-2033)
FIGURE 32 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY END USER, LIFELINE CURVE
FIGURE 33 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2025
FIGURE 34 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2026-2033 (USD THOUSAND)
FIGURE 35 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL, CAGR (2026-2033)
FIGURE 36 SWITZERLAND, FRANCE, ITALY, AND GERMANY CPAP DEVICES MARKET, BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 37 GERMANY, FRANCE, ITALY, AND SWITZERLAND CPAP DEVICES MARKET: SNAPSHOT (2025)
FIGURE 38 GERMANY, SWITZERLAND, FRANCE, ITALY CPAP DEVICES MARKET: COMPANY SHARE 2025 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。