Cholangiocarcinoma (CCA) is a heterogeneous group of malignancies that can evolve from the canals of Hering to the main bile duct. CCA are rare tumors consisting of about 3% of gastrointestinal tumors and have an overall occurrence of less than 2/100,000. They are the second most common primary hepatic malignancies following hepatocellular carcinoma (HCC). It accounts for about 20% of the deaths from hepatobiliary cancers, which cause 13% of the total cancer mortality globally. CCA is one of the most fatal cancers: although it has been seen that 1-year mortality has improved over time, the 5-year survival is still as low as 10%. The only possible treatment option for patients with CCA is surgical resection. Despite the respectability rate having been reported to be as high as 65%, curative resection rates are less than 50%. Unfortunately, two-thirds of CCA remain clinically silent and are only diagnosed in more advanced stages. At advanced stage, CCA has a devastating prognosis with a median overall survival of only 12-15 months.
Cholangiocarcinoma is a type of cancer that forms in the slender tubes that carry the digestive fluid bile. Bile ducts connect your liver to your gallbladder and to your small intestine. Cholangiocarcinoma, also known as bile duct cancer, occurs mostly in people older than 50, though it can occur at any age.
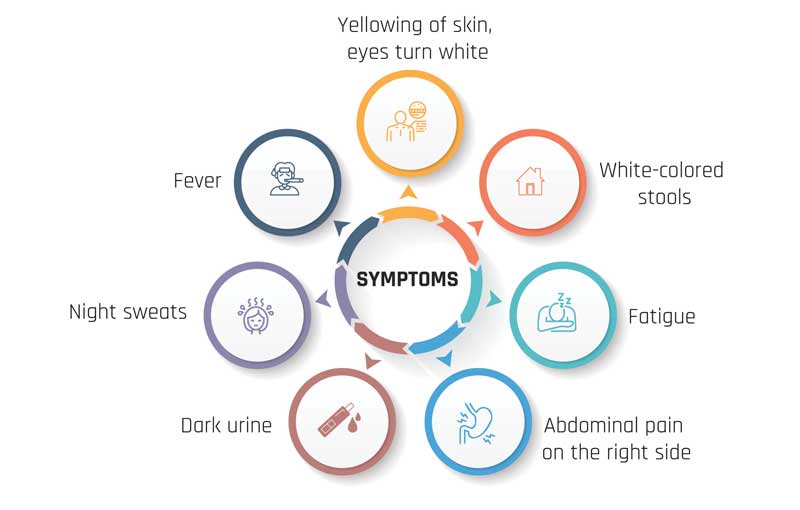
The Various Signs and Symptoms of Cholangiocarcinoma Include:
Cause of Cholangiocarcinoma
The actual reason of cholangiocarcinoma. The risk factors suggest that health conditions that cause chronic (long-term) inflammation in the bile ducts may play a role in developing this cancer. Consistent damage such as inflammation can also cause DNA changes, which may change how certain cells grow, divide and behave. These changes probably aren't inherited, which means parents don't pass them to their children. Instead, the changes are likely happen during a person's lifetime.
Symptoms and Treatment:
- Liver Function Tests- Blood tests are used to measure your liver function, indicating what's causing your signs and symptoms.
- Tumor Marker Test- Checking the level of carbohydrate antigen (CA) 19-9 in your blood may give your doctor additional clues about your diagnosis. CA 19-9 is a protein overproduced by bile duct cancer cells.
- Endoscopic Retrograde Cholangiopancreatography (ERCP)- During endoscopic retrograde cholangiopancreatography (ERCP), a thin, flexible tube equipped with a tiny camera is passed down your throat and through your digestive tract to your small intestine. The camera is used to inspect the area where your bile ducts connect to your small intestine.
- Imaging Tests- Imaging tests can help healthcare professionals see the internal organs and look for signs of cholangiocarcinoma. Techniques used to diagnose bile duct cancer include ultrasound, computerized tomography (CT) scans, and magnetic resonance imaging (MRI) combined with magnetic resonance cholangiopancreatography (MRCP)
Our DBMR team investigated the magnetic resonance imaging devices (MRI) market and witnessed that the COVID-19 epidemic had a minimal impact on the magnetic resonance imaging industry (MRI). North America dominates the magnetic resonance imaging (MRI) market because of the region's high level of advanced healthcare skills. The rising frequency of chronic diseases, the ever-growing senior population, and rising personal disposable income in Asia-Pacific are likely to drive significant growth during the projection period of 2022 to 2029.
To know more about the study, kindly visit: https://www.databridgemarketresearch.com/reports/global-magnetic-resonance-imaging-mri-devices-market
Several treatment methods include liver therapy, chemotherapy, radiation therapy, targeted drug therapy, immunotherapy. For many patients, a liver transplant can be a cure for hilar cholangiocarcinoma, but there is a high risk that the cancer will recur after a liver transplant. Radiation therapy uses high-powered energy beams from sources such as X-rays and protons to kill the cancer cells. Immunotherapy uses body's own immune system to fight cancer. Immunotherapy works by interfering with that process.
Our DBMR team investigated the chemotherapy drug market and witnessed that the COVID-19 virus has spread to nearly every country on the planet, prompting the World Health Organization (WHO) to declare it a public health emergency. North America dominates the chemotherapy drug market because of the presence of key manufacture of the product and rising geriatric population in this region. Asia-Pacific is expected to grow during the forecast period due to increased government awareness programs and rising healthcare expenditure in this region. Also, growing number of generic drugs will further cushion the market's growth rate in this region.
To know more about the study, kindly visit: https://www.databridgemarketresearch.com/reports/global-chemotherapy-drug-market
Concerning the treatment of cholangiocarcinoma, the Food and Drug Administration (FDA) recently granted accelerated approval to futibatinib for adult patients with previously treated, unrespectable, locally advanced or metastatic intrahepatic cholangiocarcinoma having fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.
This trial involved 103 patients who were previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma. All these patients received 20 mg of futibatinib orally once daily continuously for 21-day cycles until disease progression or unacceptable toxicity.
The End-Points are Discussed Below:
|
Primary
|
Secondary
|
|
Overall Response Rate(ORR)
|
Disease control rate (DCR)
Progression-free survival (PFS)
Overall survival (OS)
Safety
Patient-reported outcomes
Duration of response (DOR)
|
It was observed that the ORR was 41.7%, and the DCR was 82.5%. A complete response was observed in 1 patient, 42 patients had a partial response, 42 patients had stable disease, and 16 had progressive disease. The median duration of response was 9.5 months and the median time to response was 2.6 months. In the pre-specified subgroup analysis, the ORR was consistent across patient all subgroups.
The median progression-free survival was 8.9 months. At 6 months the PFS rate was 65%, and at 12 months it was 35%. The median OS was 20.0 months. The PFS rate at 6 months was 88% and at 12 months it was 73%.
Although, the patients received adverse reactions as well. The most common treatment-related adverse events (TRAEs) of any grade included alopecia, hyperphosphatemia, dry mouth, and diarrhea. Most TRAEs were grade 3 or less, but there were 2 grade 4 events. Grade 3 AEs Included
- Hyperphosphatemia (31%)
- Increased alanine transaminase and aspartate transaminase (12%)
- Palmar-plantar erythrodysesthesia syndrome (6%)
- Nail toxicities (2%).
Through this trial, there has been some safety and precautions noticed with this drug. It has been mentioned below:
- Ocular Toxicity: Retinal Pigment Epithelial Detachment (RPED), which may cause symptoms such as blurred vision, occurred in 9% of 318 patients who received LYTGOBI across clinical trials. The median time to first onset was 40 days. For onset of visual symptoms, one should immediately refer patients for ophthalmologic evaluation, with a regular follow-up every 3 weeks until resolution or discontinuation of LYTGOBI. In addition to this, one should withhold or reduce the dose of LYTGOBI as recommended.
- Hyperphosphatemia and Soft Tissue Mineralization: Hyperphosphatemia was reported in 88% of 318 patients treated with LYTGOBI across clinical trials with a median time of onset of 5 days. Effective monitoring must be done for hyperphosphatemia throughout treatment. One should initiate a low-phosphate diet and phosphate-lowering therapy when serum phosphate level is ≥5.5 mg/dL; phosphate-lowering therapy when >7 mg/dL; reduce dose, withhold, or permanently discontinue LYTGOBI based on duration and severity of hyperphosphatemia.
- Embryo-Fetal Toxicity: This drug can cause fetal harm. Female patients of reproductive potential, and males with female partners of reproductive potential, must use effective contraception during treatment with LYTGOBI and for 1 week post the last dose.
Conclusion:
The prevalence of cholangiocarcinoma is on the rise and thus effective treatment is necessary for the fast recovery of the patients. FDA recently approved a drug which received accelerated approval called futibatinib for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma having fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements. Such developments are necessary for treating these patients.









