Global Metabolic Dysfunction Associated Steatohepatitis Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
1.72 Billion
USD
7.04 Billion
2024
2032
USD
1.72 Billion
USD
7.04 Billion
2024
2032
| 2025 –2032 | |
| USD 1.72 Billion | |
| USD 7.04 Billion | |
|
|
|
Global Metabolic Dysfunction-associated Steatohepatitis Market Segmentation, By Therapeutic Targets (Farnesoid X receptor, Peroxisome proliferator activated receptor, and FGF-21), Treatment (Drug, Rezdiffra, Therapy, Phytic Acid Based Nano-Medicine Therapy, Weight Management, Diabetes Control, Regular Exercise, and Others), End-User (Hospitals, Specialty Clinics, Homecare, and Others) – Industry Trends and Forecast to 2032
Metabolic Dysfunction-associated Steatohepatitis Market Analysis
The Metabolic Dysfunction-associated Steatohepatitis (MASH) market is witnessing significant growth driven by increasing awareness of metabolic diseases, rising healthcare expenditures, and advancements in diagnostics and treatments. MASH, as a complex condition linked to metabolic dysfunction, has attracted attention from pharmaceutical companies and researchers, leading to the development of targeted therapies and novel drugs. The market is characterized by the presence of both established pharmaceutical giants and emerging biotech companies working on innovative therapies aimed at addressing the underlying causes of the disease, such as insulin resistance, inflammation, and liver fibrosis.
A key factor driving the market is the high unmet need for effective treatments, as current options are limited and primarily focus on symptom management rather than disease reversal. The growing focus on personalized medicine is also playing a role in advancing the MASH treatment landscape. Furthermore, increasing research and development investments are expected to accelerate the introduction of new therapies in the pipeline. Despite challenges such as regulatory hurdles and the complexity of the disease, the market for MASH is poised for growth. As the disease burden rises globally, the demand for advanced and effective treatment solutions will likely continue to escalate, positioning MASH as an area of focus for medical innovation in the coming years.
Metabolic Dysfunction-associated Steatohepatitis Market Size
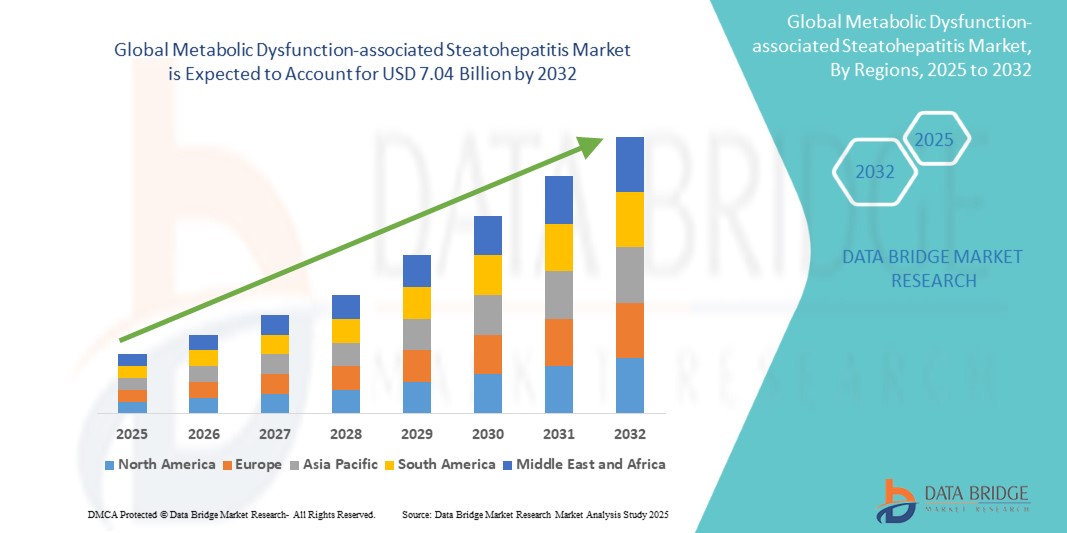
The global Metabolic Dysfunction-associated Steatohepatitis market size was valued at USD 1.72 billion in 2024 and is projected to reach USD 7.04 billion by 2032, with a CAGR of 19.23% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Metabolic Dysfunction-associated Steatohepatitis Market Trends
“Growing Focus on Developing Non-Invasive Diagnostic Tools”
One prominent trend in the Metabolic Dysfunction-associated Steatohepatitis (MASH) market is the growing focus on developing non-invasive diagnostic tools. Traditional diagnostic methods, such as liver biopsy, are invasive, costly, and carry risks, which has led to an increasing demand for alternative approaches. Companies are investing heavily in technologies such as biomarkers, imaging techniques, and blood tests that can accurately diagnose MASH without the need for invasive procedures. This trend is driven by the desire for earlier diagnosis, better patient monitoring, and improved patient outcomes. Non-invasive diagnostics also allow for more frequent screening, which is essential as the prevalence of MASH is rising with the global increase in metabolic disorders. With the ongoing advancements in artificial intelligence and machine learning, the development of highly sensitive and specific diagnostic tools is expected to further streamline MASH detection, making it more accessible and cost-effective for healthcare providers and patients alike.
Report Scope and Metabolic Dysfunction-associated Steatohepatitis Market Segmentation
|
Attributes |
Metabolic Dysfunction-associated Steatohepatitis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
89bio, Inc. (U.S.), Altimmune (U.S.), Alnylam Pharmaceuticals, Inc. (U.S.), Alimentiv Inc. (Canada), Arrowhead Pharmaceuticals Inc. (U.S.), Boehringer Ingelheim International GmbH (Germany), Cellarity (U.S.), Corcept Therapeutics, Incorporated (U.S.), Gilead Sciences, Inc. (U.S.), Galectin Therapeutics, Inc. (U.S.), Ionis Pharmaceuticals (U.S.), Intercept Pharmaceuticals, Inc. (U.S.), Lilly (U.S.), Madrigal Pharmaceuticals (U.S.), Merck & Co., Inc. (U.S.), Novo Nordisk A/S (Denmark), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Regeneron Pharmaceuticals Inc. (U.S.) and Sagimet Biosciences Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Metabolic Dysfunction-associated Steatohepatitis Market Definition
Metabolic Dysfunction-associated Steatohepatitis (MASH) is a liver condition characterized by inflammation, fat accumulation, and liver damage associated with metabolic dysfunction, such as obesity, insulin resistance, and type 2 diabetes. MASH is considered a more advanced form of non-alcoholic fatty liver disease (NAFLD), where the fat buildup in the liver leads to inflammation and potentially progresses to more severe liver conditions, including cirrhosis and liver cancer. The term "MASH" reflects the growing recognition that metabolic factors, rather than alcohol consumption, play a primary role in the development and progression of liver disease.
Metabolic Dysfunction-associated Steatohepatitis Market Dynamics
Drivers
- Growing Awareness and Diagnosis of MASH
As awareness of Metabolic Dysfunction-associated Steatohepatitis (MASH) increases, there has been a surge in the identification and diagnosis of the disease. In recent years, healthcare providers have become more proficient in recognizing the condition, which was previously underdiagnosed due to its asymptomatic nature in early stages. With improved diagnostic techniques, such as advanced imaging and non-invasive biomarkers, more patients are being diagnosed earlier in the disease progression. This trend is particularly evident in developed countries, where healthcare infrastructure supports better screening for metabolic diseases. For instance, non-alcoholic fatty liver disease (NAFLD), which often progresses to MASH, is now increasingly recognized as a major health issue. Early detection of MASH has created a significant demand for treatment options, further driving the market as more patients seek therapeutic interventions.
- Regulatory Approvals and Market Access
Recent regulatory approvals for MASH treatments have greatly impacted the market’s growth. As more drug candidates move through clinical trials and gain approval, the market for MASH therapies expands. For instance, the approval of the drug obeticholic acid for treating liver diseases related to metabolic dysfunction has provided a new therapeutic option for patients. Such regulatory milestones not only validate the potential of MASH treatments but also encourage further investment and development in the field. These approvals make treatments more accessible to patients, improving market penetration and increasing overall market growth. With regulatory bodies such as the FDA prioritizing treatments for metabolic diseases, the ease of market entry for new therapies is improving, ensuring the continuous growth of the MASH treatment market.
Opportunities
- Expansion of Personalized Medicine
The increasing emphasis on personalized medicine presents a significant opportunity for the Metabolic Dysfunction-associated Steatohepatitis (MASH) market. Personalized medicine, which tailors treatments based on an individual’s genetic profile, lifestyle, and disease progression, can greatly enhance the efficacy of MASH therapies. For instance, genetic testing could help identify patients who are most likely to benefit from specific treatments, such as novel FXR agonists or metabolic modulators. As precision medicine becomes more mainstream, pharmaceutical companies are focusing on developing drugs that can be customized to meet individual patient needs. This approach not only offers a more targeted treatment option but also improves patient outcomes. By expanding the range of personalized treatments, this trend could lead to higher treatment adoption rates, driving market growth as more patients benefit from tailored therapies that maximize efficacy and minimize side effects.
- Emerging Markets and Rising Healthcare Investment
Emerging markets present a vast opportunity for the MASH market due to growing healthcare investment and an increase in the prevalence of metabolic disorders. As countries in Asia, Africa, and Latin America experience rapid urbanization, changes in diet, and a rise in sedentary lifestyles, the rates of obesity and type 2 diabetes are climbing, increasing the incidence of MASH. Governments and healthcare organizations in these regions are investing heavily in improving healthcare infrastructure, which includes expanding access to diagnostics and treatments for liver diseases. For instance, countries such as China and India are seeing a surge in demand for non-alcoholic fatty liver disease (NAFLD) diagnostics and treatments, creating opportunities for pharmaceutical companies to expand their presence. With increasing economic development and healthcare funding, emerging markets are expected to significantly contribute to the MASH market's growth, as more patients gain access to therapies and advanced care.
Restraints/Challenges
- High Cost and Complexity of Treatment Development
One key restraint in the Metabolic Dysfunction-associated Steatohepatitis market is the high cost and complexity of treatment development. MASH, a liver condition linked to metabolic diseases such as obesity and type 2 diabetes, has no universally approved drug therapies yet. The treatment pipeline involves complex drug discovery, clinical trials, and regulatory approvals, all of which are time-consuming and costly. In addition, the lack of standardized diagnostic criteria for MASH makes it challenging to identify and treat patients consistently. For instance, drug candidates such as obeticholic acid and elafibranor, designed for non-alcoholic steatohepatitis (NASH), face delays in FDA approvals due to mixed clinical trial results. This can significantly hinder the speed at which effective therapies reach the market. The impact of this challenge on market growth is significant, as delays in therapy approval and development increase uncertainty, reducing investor confidence and hindering market expansion.
- Complex Disease Pathophysiology
The complex and multifactorial nature of Metabolic Dysfunction-associated Steatohepatitis represents a significant challenge in the market. MASH involves intricate interactions between metabolic dysfunction, genetic factors, and environmental triggers, making it difficult to develop a one-size-fits-all treatment. For instance, patients with varying degrees of liver damage or underlying conditions such as obesity and insulin resistance may respond differently to the same therapy. The variability in patient response complicates both the development and clinical adoption of effective treatments. In addition, the lack of a universally accepted diagnostic criteria for MASH further complicates its detection and treatment. As a result, pharmaceutical companies face challenges in creating targeted therapies that address the disease in all its forms. This complexity slows down the development of comprehensive treatment solutions, which in turn impacts market growth by delaying product launches and limiting patient access to effective therapies.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Metabolic Dysfunction-associated Steatohepatitis Market Scope
The market is segmented on the basis of therapeutic targets, treatment, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Therapeutic Targets
- Farnesoid X Receptor
- Peroxisome Proliferator Activated Receptor
- FGF-21
Treatment
- Drug
- Rezdiffra
- Therapy
- Phytic Acid Based Nano-Medicine Therapy
- Weight Management
- Diabetes Control
- Regular Exercise
- Others
End-User
- Hospitals
- Specialty Clinics
- Homecare
- Others
Metabolic Dysfunction-associated Steatohepatitis Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, therapeutic targets, treatment, and end-user as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Metabolic Dysfunction-associated Steatohepatitis (MASH) market due to the high prevalence of metabolic disorders such as obesity and type 2 diabetes. The region benefits from advanced healthcare infrastructure, widespread awareness, and strong research and development activities. In addition, the presence of key pharmaceutical companies and ongoing clinical trials for MASH treatments further accelerates market growth. The U.S., in particular, has a significant patient population, driving demand for innovative diagnostic tools and therapeutic solutions.
Asia-Pacific is expected to exhibit the highest growth rate in the Metabolic Dysfunction-associated Steatohepatitis (MASH) market. The region is experiencing a rapid increase in metabolic disorders due to changing diets, urbanization, and sedentary lifestyles. Countries such as China and India are witnessing rising obesity rates, leading to a higher incidence of MASH. With improving healthcare infrastructure, growing awareness, and increasing investments in medical research, Asia-Pacific presents significant opportunities for market expansion and is expected to see substantial growth in the coming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Metabolic Dysfunction-associated Steatohepatitis Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Metabolic Dysfunction-associated Steatohepatitis Market Leaders Operating in the Market Are:
- 89bio, Inc. (U.S.)
- Altimmune (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Alimentiv Inc. (Canada)
- Arrowhead Pharmaceuticals Inc. (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Cellarity (U.S.)
- Corcept Therapeutics, Incorporated (U.S.)
- Gilead Sciences, Inc. (U.S.)
- Galectin Therapeutics, Inc. (U.S.)
- Ionis Pharmaceuticals (U.S.)
- Intercept Pharmaceuticals, Inc. (U.S.)
- Lilly (U.S.)
- Madrigal Pharmaceuticals (U.S.)
- Merck & Co., Inc. (U.S.)
- Novo Nordisk A/S (Denmark)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Sagimet Biosciences Inc. (U.S.)
Latest Developments in Metabolic Dysfunction-associated Steatohepatitis Market
- In November 2024, Novo Nordisk announced the key findings from part 1 of the ongoing ESSENCE trial, a pivotal phase 3, 240-week, double-blind study involving 1,200 adults with metabolic dysfunction-associated steatohepatitis (MASH) and moderate to advanced liver fibrosis (stages 2 or 3). Part 1 of the trial assessed the impact of once-weekly semaglutide 2.4 mg on liver tissue (histology) compared to a placebo, alongside standard care, for the first 800 participants over 72 weeks. Novo Nordisk plans to seek regulatory approvals in the US and EU in the first half of 2025
- In June 2024, Eli Lilly and Company announced the detailed results from the SYNERGY-NASH Phase 2 study, which involved 190 patients, with or without type 2 diabetes, to evaluate the investigational use of tirzepatide in adults with biopsy-confirmed metabolic dysfunction-associated steatohepatitis (MASH) and stage 2 or 3 fibrosis. The efficacy results revealed the achievement of absence of MASH with no worsening of fibrosis on liver histology, compared to 13.2% in the placebo group after 52 weeks of treatment. This outcome met the study’s primary endpoint
- In March 2024, Madrigal Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Rezdiffra (resmetirom) as an adjunct to diet and exercise for treating adults with non-cirrhotic NASH and moderate to advanced liver fibrosis (stages F2 to F3 fibrosis). NASH, also referred to as metabolic dysfunction-associated steatohepatitis (MASH), is a liver disease characterized by inflammation and fat buildup
- In March 2024, Ionis Pharmaceuticals, Inc. announced positive results from a Phase 2 study of ION224, an investigational DGAT2 antisense inhibitor being developed for the treatment of metabolic dysfunction-associated steatohepatitis (MASH), previously known as nonalcoholic steatohepatitis (NASH). The study successfully met its primary endpoint at both the 120 mg and 90 mg doses, demonstrating liver histological improvement. In addition, it achieved the key secondary endpoint of MASH resolution
- In February 2024, Boehringer Ingelheim announced that up to 83.0% of adults treated with survodutide (BI 456906) demonstrated a statistically significant improvement in metabolic dysfunction-associated steatohepatitis (MASH) compared to 18.2% in the placebo group in a Phase II trial. primary endpoint, with survodutide showing a biopsy-confirmed improvement in MASH The trial achieved its after 48 weeks, without worsening fibrosis in stages F1, F2, and F3 (mild to moderate or advanced scarring. Survodutide has the potential to become a leading treatment for MASH, a liver disease linked to cardiovascular, renal, and metabolic conditions
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.