Mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África, por tipo de prueba (imágenes, biopsia, inmunohistoquímica, biomarcador, prueba genética , citogenética, punción lumbar, análisis de sangre, citoquímica y otros), estadio del cáncer (estadio IV, estadio III, estadio II, estadio I y estadio 0), tipo de tumor (linfomas agresivos y linfomas indolentes), producto (productos basados en instrumentos, productos basados en plataformas, kits y reactivos y otros consumibles), tecnología (hibridación in situ fluorescente, secuenciación de próxima generación, fluorinmunoensayo, hibridación genómica comparativa, inmunohistoquímica y otros), aplicación (detección, diagnóstico y predicción, pronóstico e investigación), usuario final (hospitales, centros de diagnóstico, centros de investigación del cáncer, institutos académicos, centros quirúrgicos ambulatorios y otros), canal de distribución (licitación directa, ventas minoristas y otros) - Tendencias de la industria y Pronóstico hasta 2030.
Análisis y perspectivas del mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África
La creciente conciencia sobre el linfoma no Hodgkin a nivel mundial ha aumentado la demanda del mercado. El aumento del gasto sanitario para mejorar los servicios sanitarios también contribuye al crecimiento del mercado. Los principales actores del mercado se centran en diversos lanzamientos y aprobaciones de servicios durante este período crucial. Además, el aumento de los procesos y técnicas de diagnóstico mejorados también contribuye a la creciente demanda de pruebas de diagnóstico del linfoma no Hodgkin.
Se espera que el mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África crezca en el año de pronóstico debido al aumento de los actores del mercado y la disponibilidad de servicios avanzados. Junto con esto, los fabricantes están involucrados en actividades de I+D para lanzar nuevos servicios en el mercado. La creciente investigación en el campo del diagnóstico y desarrollo del linfoma no Hodgkin está impulsando aún más el crecimiento del mercado. Sin embargo, las dificultades en las técnicas de detección del linfoma no Hodgkin podrían obstaculizar el crecimiento del mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África en el período de pronóstico.
Se espera que el aumento del gasto sanitario en el diagnóstico y tratamiento del cáncer genere oportunidades para que el mercado mejore el tratamiento. Sin embargo, el alto coste de las pruebas y las estrictas normas y regulaciones para la aprobación y comercialización de productos de diagnóstico del cáncer pueden suponer un reto para el crecimiento del mercado.
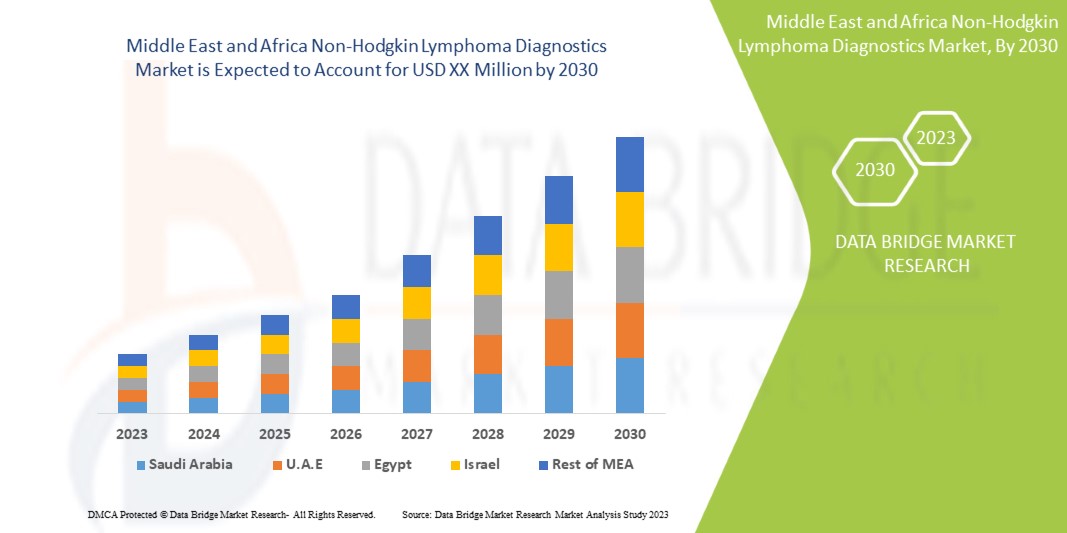
El mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África es favorable y tiene como objetivo reducir la progresión de la enfermedad. Data Bridge Market Research analiza que el mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África crecerá a una tasa compuesta anual del 8,5 % durante el período de pronóstico de 2023 a 2030.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2015 - 2020) |
|
Unidades cuantitativas |
Ingresos en millones de USD y precios en USD |
|
Segmentos cubiertos |
Tipo de prueba (imágenes, biopsia, inmunohistoquímica, biomarcador, prueba genética, citogenética, punción lumbar, análisis de sangre, citoquímica y otros), estadio del cáncer (estadio IV, estadio III, estadio II, estadio I y estadio 0), tipo de tumor (linfomas agresivos y linfomas indolentes), producto (productos basados en instrumentos, productos basados en plataformas, kits y reactivos y otros consumibles), tecnología (hibridación in situ fluorescente, secuenciación de próxima generación, fluorinmunoensayo, hibridación genómica comparativa, inmunohistoquímica y otros), aplicación (cribado, diagnóstico y predicción, pronóstico e investigación), usuario final (hospitales, centros de diagnóstico, centros de investigación del cáncer, institutos académicos, centros quirúrgicos ambulatorios y otros), canal de distribución (licitación directa, ventas minoristas y otros) |
|
Países cubiertos |
Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Egipto, Israel, Kuwait, resto de Oriente Medio y África. |
|
Actores del mercado cubiertos |
CANON MEDICAL SYSTEMS CORPORATION (Prefectura de Tochigi, Japón), Koninklijke Philips NV (Ámsterdam, Países Bajos), Siemens Healthcare GmbH (Erlangen, Alemania), Danaher (Washington, EE. UU.), Bio-Rad Laboratories, Inc (California, EE. UU.), General Electric Company (Massachusetts, EE. UU.), Sysmex Corporation (Hyogo, Japón), Grail (California, EE. UU.), F. Hoffmann-La Roche (Basilea, Suiza), Neusoft Corporation (Shenyang, China), Agilent Technologies, Inc. (California, EE. UU.), NeoGenomics Laboratories (Florida, EE. UU.), Hologic, Inc (Massachusetts, EE. UU.), Integrated DNA Technologies, Inc., CENTOGENE NV (Rostock, Alemania), Merit Medical Systems (Utah, EE. UU.), Invitae Corporation (California, EE. UU.), PerkinElmer Inc. (Massachusetts, EE. UU.), QIAGEN (California, EE. UU.) y GeneDx, LLC (Connecticut, EE. UU.), entre otros. |
Definición de mercado
El cáncer comienza en los ganglios linfáticos y se presenta con mayor frecuencia en personas que fuman. Los dos tipos principales de linfoma no Hodgkin son el linfoma no Hodgkin agresivo y el linfoma no Hodgkin indolente. Las causas del linfoma no Hodgkin incluyen el tabaquismo, el tabaquismo pasivo, la exposición a ciertas toxinas y los antecedentes familiares.
Los síntomas incluyen tos (a menudo con sangre), dolor en el pecho, sibilancia y pérdida de peso. Estos síntomas no suelen aparecer hasta que el cáncer está avanzado. Los tratamientos varían, pero pueden incluir cirugía, quimioterapia, radioterapia, terapia farmacológica dirigida e inmunoterapia.
Dinámica del mercado de diagnóstico del linfoma no Hodgkin en Oriente Medio y África
En esta sección se aborda la comprensión de los factores impulsores del mercado, las ventajas, las oportunidades, las limitaciones y los desafíos. Todo esto se analiza en detalle a continuación:
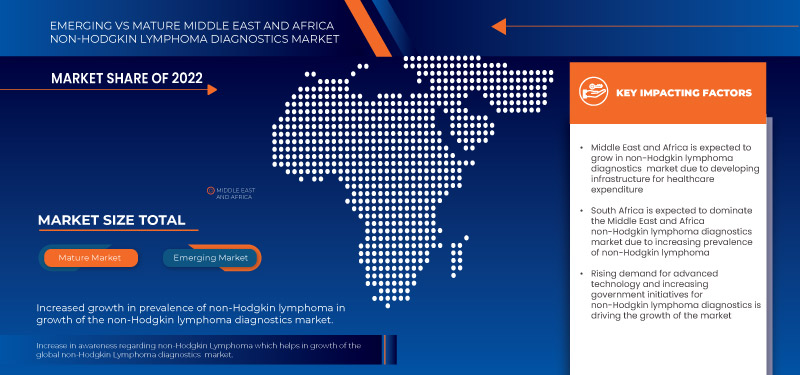
- Aumento del crecimiento de la prevalencia del linfoma no Hodgkin
El linfoma es el nombre general de muchos subtipos relacionados de cáncer que surgen de un tipo de glóbulo blanco llamado "linfocito". El linfoma se divide en dos categorías principales: linfoma de Hodgkin (LH) y linfoma no Hodgkin (LNH). El linfoma no Hodgkin es una categoría general de linfoma. Hay muchos subtipos que caen dentro de esta categoría. El linfoma difuso de células B grandes y el linfoma folicular se encuentran entre los subtipos más comunes. Los casos de cáncer de linfoma están aumentando en todo el mundo. El linfoma no Hodgkin (LNH) es uno de los cánceres más comunes en los Estados Unidos, representando aproximadamente el 4% de todos los cánceres.
La probabilidad de que un hombre desarrolle LNH durante su vida es de aproximadamente 1 en 43; para una mujer, el riesgo es de aproximadamente 1 en 53. En 2020, según la Agencia Internacional para la Investigación sobre el Cáncer de la Organización Mundial de la Salud (OMS), se estimó que 544.352 personas fueron diagnosticadas con linfoma no Hodgkin en todo el mundo.
- Aumenta la conciencia sobre el linfoma no Hodgkin
La concientización sobre el linfoma no Hodgkin es una oportunidad para aumentar el conocimiento sobre estas enfermedades y poner de relieve la investigación sobre sus causas, prevención, diagnóstico, tratamiento y supervivencia. El objetivo es ayudar a las personas afectadas por el linfoma y promover hábitos saludables.
El quinto cáncer más frecuente en el mundo es el linfoma. Según el informe de la Organización Mundial de la Salud, Asia tiene la tasa de incidencia más alta, con 241.270 pacientes en los últimos 5 años. Además, el linfoma no Hodgkin representa el 7,4% de todas las muertes por cáncer en Asia. La tasa de mortalidad ha aumentado muy lentamente cada año.
Cada año, se planifican varias campañas de concienciación, seminarios web y conferencias en todo el mundo para educar a las personas sobre el linfoma no Hodgkin, y varias organizaciones toman la iniciativa en estas campañas. Como resultado, se espera que esto impulse el crecimiento del mercado de diagnóstico del linfoma no Hodgkin en Oriente Medio y África.
- Falta de disponibilidad de profesionales médicos capacitados y calificados
Se estima que la densidad de la fuerza laboral total del sector de la salud es de 29 por cada 10.000 habitantes, pero solo de 16 por cada 10.000 trabajadores capacitados. La necesidad de profesionales calificados y certificados es una gran limitación para el mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África. La demanda de hibridación in situ fluorescente ha aumentado debido al aumento de casos de enfermedades cancerosas y anomalías genéticas, pero se espera que la menor cantidad de profesionales capacitados presentes obstaculice el crecimiento del mercado.
La capacidad de un médico cualificado para realizar numerosas pruebas es fundamental para el crecimiento de los sectores sanitario y médico. El objetivo principal de contar con un equipo profesional es garantizar que todos los equipos médicos que salvan vidas funcionen de forma eficaz cuando más se los necesita. El técnico debe tener la formación y la certificación adecuadas de los fabricantes y las autoridades pertinentes.
Los técnicos enfrentan brechas de capacitación técnica relacionadas con problemas para adaptar métodos avanzados de manera segura para realizar procedimientos de manera eficiente. Para el diagnóstico del linfoma, existe una gran necesidad de profesionales capacitados para actividades de desarrollo, validación, operación y resolución de problemas de métodos.
- Normas y directrices estrictas para diferentes tratamientos y diagnósticos
La venta de dispositivos médicos o medicamentos en dichas jurisdicciones puede suponer un gasto financiero sustancial, que podría llevar meses o años. Si no se comprenden o no se tienen en cuenta estas limitaciones, las demoras pueden poner en grave peligro la probabilidad de éxito en un mercado altamente competitivo. Pero su aprobación y comercialización en múltiples regiones del mundo exige el cumplimiento de normas regulatorias estrictas y la aceptación de diversos organismos reguladores.
Las empresas deben revisar cuidadosamente las especificaciones de los productos antes de clasificarlos según los protocolos de la Administración de Alimentos y Medicamentos (FDA). Establecer programas de descubrimiento y desarrollo de fármacos en empresas académicas, sin fines de lucro y de otras áreas de investigación en ciencias de la vida requiere una planificación cuidadosa. Cada país tiene su propio organismo regulador, y las agencias reguladoras tienen una supervisión mínima de los estudios de descubrimiento de fármacos, pero proporcionan ciertos requisitos y documentación clara para lograr hitos durante este proceso. El marco regulatorio tiene como objetivo mejorar el acceso para una mejor calidad y brindar orientación sobre el fortalecimiento de los controles regulatorios.
Impacto posterior a la COVID-19 en el mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África
La elevada carga de COVID-19 en los sistemas de atención sanitaria de todo el mundo ha suscitado inquietudes entre los oncólogos médicos sobre el impacto de COVID-19 en el diagnóstico y el tratamiento del linfoma no Hodgkin. Investigamos el impacto de COVID-19 en el diagnóstico y el tratamiento del linfoma no Hodgkin antes y después de la era de COVID-19 en este estudio de cohorte retrospectivo. Durante la pandemia, los nuevos diagnósticos de linfoma no Hodgkin disminuyeron a medida que se presentaban estadios ligeramente más avanzados de la enfermedad, y hubo un aumento significativo de la radiocirugía como primer tratamiento definitivo y una disminución tanto del tratamiento sistémico como de la cirugía en comparación con la era anterior a COVID-19. En comparación con la época anterior a COVID-19, no hubo un retraso significativo en el inicio de la quimioterapia y la radioterapia durante la pandemia.
Sin embargo, durante la pandemia, observamos un retraso en la cirugía del linfoma no Hodgkin. La COVID-19 parece haber tenido un impacto significativo en los diagnósticos y los patrones de tratamiento de los pacientes con linfoma no Hodgkin en nuestro centro de linfoma no Hodgkin. A muchos oncólogos les preocupa que el número de pacientes con linfoma no Hodgkin recién diagnosticados aumente el próximo año. Esta investigación aún está en curso y se recopilará y analizará más información para comprender mejor el impacto general de la pandemia de COVID-19 en nuestra población de pacientes con linfoma no Hodgkin.
Desarrollo reciente
- En noviembre de 2022, Danaher anunció que había firmado una asociación estratégica con la Universidad de Duke para formar su primer programa Danaher Beacon for Gene Therapy Innovation. El programa invierte en innovación de productos para impulsar estrategias externas de I+D con un enfoque en medicamentos genómicos, diagnósticos de precisión, biofabricación de próxima generación, sistemas humanos y ciencias de datos.
- En agosto de 2022, Bio-Rad Laboratories Inc. adquirió Curiosity Diagnostics, una empresa con sede en Polonia que desarrolla soluciones tecnológicas innovadoras para los mercados de diagnóstico médico y atención sanitaria. Esto ha ayudado a la empresa a aumentar su cartera de productos y su presencia en el mercado de Oriente Medio y África.
Segmentación del mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África
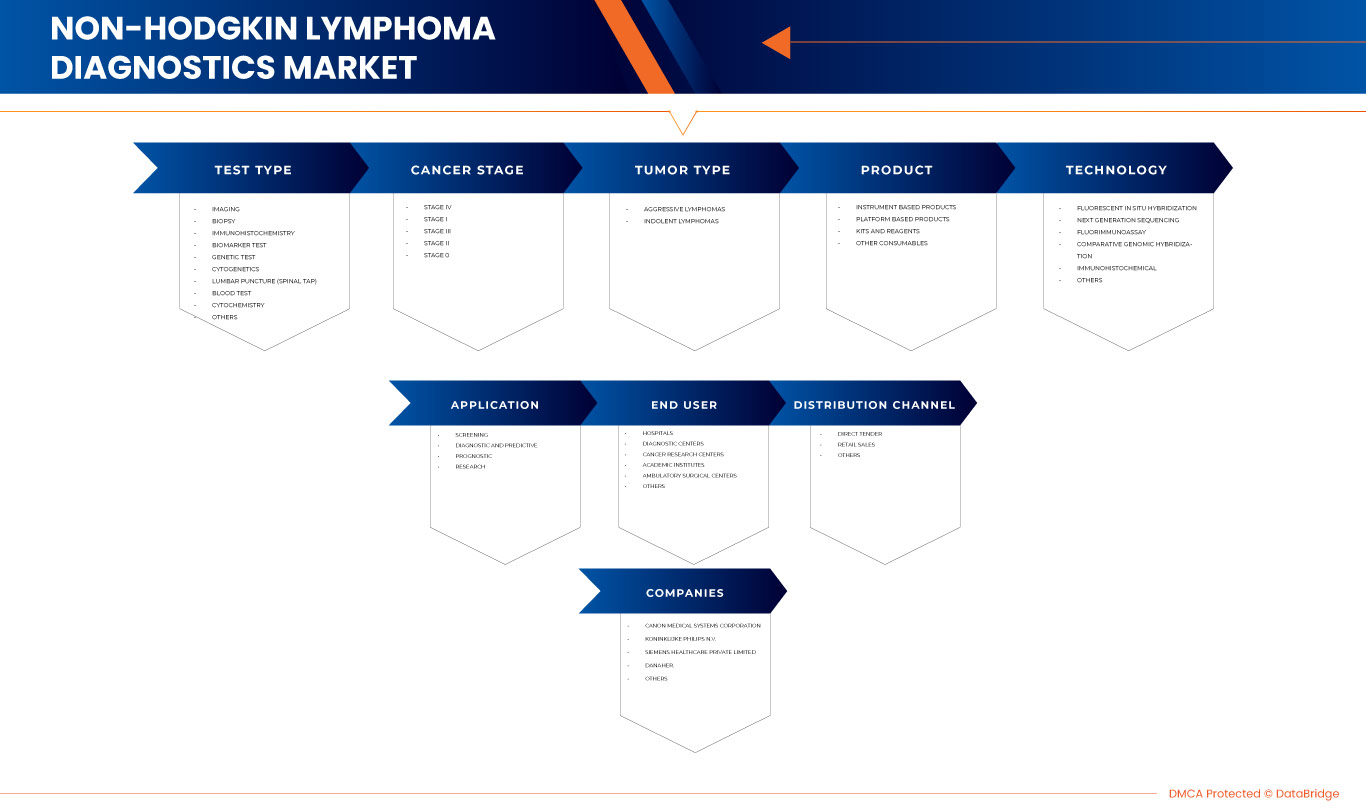
El mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África se clasifica en ocho segmentos notables, que se basan en el tipo de prueba, el estadio del cáncer, el tipo de tumor, el producto, la tecnología, la aplicación, los usuarios finales y el canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
POR TIPO DE PRUEBA
- Imágenes
- Biopsia
- Inmunohistoquímica
- Prueba de biomarcadores
- Prueba genética
- Citogenética
- Punción lumbar (punción espinal)
- Análisis de sangre
- Citoquímica
- Otros
POR ETAPA DEL CÁNCER
- Etapa 0
- Etapa I
- Estadio II
- Estadio III
- Estadio IV
POR TIPO DE TUMOR
- Linfomas agresivos
- Linfomas indolentes
POR PRODUCTO
- Productos basados en instrumentos
- Productos basados en plataformas
- Kits y reactivos
- Otros consumibles
POR TECNOLOGÍA
- Hibridación fluorescente in situ
- Secuenciación de próxima generación
- Inmunoensayo fluorográfico
- Hibridación genómica comparativa
- Inmunohistoquímica
- Otros
POR APLICACIÓN
- Cribado
- Diagnóstico y predictivo
- Pronóstico
- Investigación
POR USUARIO FINAL
- Hospitales
- Centros de diagnóstico
- Centros de investigación del cáncer
- Institutos académicos
- Centros de cirugía ambulatoria
- Otros
POR CANAL DE DISTRIBUCIÓN
- Licitación directa
- Ventas al por menor
- Otros
Análisis y perspectivas regionales del mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África
Se analiza el mercado de diagnóstico de linfoma no Hodgkin de Medio Oriente y África, y se proporcionan información y tendencias del tamaño del mercado por tipo de prueba, estadio del cáncer, tipo de tumor, producto, tecnología, aplicación, usuario final y canal de distribución como se mencionó anteriormente.
Los países cubiertos en el informe de diagnóstico de linfoma no Hodgkin son Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Egipto, Israel, Kuwait, el resto de Oriente Medio y África.
Sudáfrica domina el mercado de diagnóstico de linfoma no Hodgkin en Medio Oriente y África debido al conocimiento exponencial del diagnóstico del cáncer y los servicios de consultoría en el país.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en la regulación del mercado que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como el análisis de la cadena de valor ascendente y descendente, las tendencias técnicas y el análisis de las cinco fuerzas de Porter, y los estudios de casos son algunos de los indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de Medio Oriente y África y sus desafíos afrontados debido a la gran o escasa competencia de las marcas locales y nacionales, el impacto de los aranceles nacionales y las rutas comerciales se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis de la cuota de mercado de diagnóstico del linfoma no Hodgkin en Oriente Medio y África y panorama competitivo
El panorama competitivo del mercado de diagnóstico de linfoma no Hodgkin en Oriente Medio y África proporciona detalles de los competidores. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia en Oriente Medio y África, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y la extensión de los productos y el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en el mercado de diagnóstico de linfoma no Hodgkin.
Algunos de los principales actores que operan en el mercado son CANON MEDICAL SYSTEMS CORPORATION, Koninklijke Philips NV, Siemens Healthcare GmbH, Danaher., Bio-Rad Laboratories, Inc., General Electric Company, Sysmex Corporation, Grail, F. Hoffmann-La Roche, Neusoft Corporation, Agilent Technologies, Inc., NeoGenomics Laboratories, Hologic, Inc, Integrated DNA Technologies, Inc., CENTOGENE NV, Merit Medical Systems, Invitae Corporation, PerkinElmer Inc., QIAGEN y GeneDx, LLC, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
5 INDUSTRY INSIGHTS
6 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 INCREASED GROWTH IN PREVALENCE OF NON-HODGKIN LYMPHOMA
7.1.2 INCREASE IN AWARENESS REGARDING NON-HODGKIN LYMPHOMA
7.1.3 ADVANCEMENT IN ARTIFICIAL INTELLIGENCE IN DIAGNOSIS OF NON-HODGKIN LYMPHOMA
7.1.4 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UP
7.2 RESTRAINTS
7.2.1 LACK OF AVAILABILITY OF TRAINED AND SKILLED MEDICAL PROFESSIONALS
7.2.2 STRINGENT REGULATIONS AND GUIDELINES FOR DIFFERENT TREATMENTS AND DIAGNOSIS
7.3 OPPORTUNITIES
7.3.1 TECHNICAL ADVANCEMENTS IN CANCER DIAGNOSIS
7.3.2 INCREASING HEALTHCARE EXPENDITURE IN CANCER R&D
7.3.3 GROWING INITIATIVES BY GOVERNMENT AND KEY PLAYERS
7.4 CHALLENGES
7.4.1 LACK OF EARLY DIAGNOSTIC AWARENESS AMONG PEOPLE
7.4.2 HIGH DIAGNOSTIC COST AND FEAR OF TREATMENT
7.4.3 COMMON MISDIAGNOSIS OF NON-HODGKIN LYMPHOMA (NHL)
8 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 IMAGING
8.2.1 COMPUTED TOMOGRAPHY (CT)
8.2.2 CHEST X-RAY
8.2.3 MAGNETIC RESONANCE IMAGING (MRI)
8.2.4 ULTRASOUND
8.2.5 POSITRON EMISSION TOMOGRAPHY (PET)
8.3 BIOPSY
8.3.1 EXCISIONAL OR INCISIONAL BIOPSY
8.3.2 CORE NEEDLE BIOPSY
8.4 IMMUNOHISTOCHEMISTRY
8.5 BIOMARKER TEST
8.5.1 BETA 2-M
8.5.2 LDH
8.5.3 CA-125
8.5.4 TP53
8.5.5 NPM1
8.5.6 OTHERS
8.6 GENETIC TEST
8.7 CYTOGENETICS
8.8 LUMBAR PUNCTURE (SPINAL TAP)
8.9 BLOOD TEST
8.1 CYTOCHEMISTRY
8.11 OTHERS
9 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY TUMOR TYPE
9.1 OVERVIEW
9.2 AGGRESSIVE LYMPHOMAS
9.2.1 DIFFUSE LARGE B CELL LYMPHOMA
9.2.1.1 INSTRUMENT BASED PRODUCTS
9.2.1.2 PLATFORM BASED PRODUCTS
9.2.1.3 KITS AND REAGENTS
9.2.1.4 OTHER CONSUMABLES
9.2.2 ANAPLASTIC LARGE-CELL LYMPHOMA
9.2.2.1 INSTRUMENT BASED PRODUCTS
9.2.2.2 PLATFORM BASED PRODUCTS
9.2.2.3 KITS AND REAGENTS
9.2.2.4 OTHER CONSUMABLES
9.2.3 MANTLE CELL LYMPHOMA
9.2.3.1 INSTRUMENT BASED PRODUCTS
9.2.3.2 PLATFORM BASED PRODUCTS
9.2.3.3 KITS AND REAGENTS
9.2.3.4 OTHER CONSUMABLES
9.2.4 PERIPHERAL T-CELL LYMPHOMA
9.2.4.1 INSTRUMENT BASED PRODUCTS
9.2.4.2 PLATFORM BASED PRODUCTS
9.2.4.3 KITS AND REAGENTS
9.2.4.4 OTHER CONSUMABLES
9.2.5 LYMPHOBLASTIC LYMPHOMA
9.2.5.1 INSTRUMENT BASED PRODUCTS
9.2.5.2 PLATFORM BASED PRODUCTS
9.2.5.3 KITS AND REAGENTS
9.2.5.4 OTHER CONSUMABLES
9.2.6 BURKITT LYMPHOMA
9.2.6.1 INSTRUMENT BASED PRODUCTS
9.2.6.2 PLATFORM BASED PRODUCTS
9.2.6.3 KITS AND REAGENTS
9.2.6.4 OTHER CONSUMABLES
9.3 INDOLENT LYMPHOMAS
9.3.1 FOLLICULAR LYMPHOMA
9.3.1.1 INSTRUMENT BASED PRODUCTS
9.3.1.2 PLATFORM BASED PRODUCTS
9.3.1.3 KITS AND REAGENTS
9.3.1.4 OTHER CONSUMABLES
9.3.2 CUTANEOUS T-CELL LYMPHOMA
9.3.2.1 INSTRUMENT BASED PRODUCTS
9.3.2.2 PLATFORM BASED PRODUCTS
9.3.2.3 KITS AND REAGENTS
9.3.2.4 OTHER CONSUMABLES
9.3.3 MARGINAL ZONE B CELL LYMPHOMA
9.3.3.1 INSTRUMENT BASED PRODUCTS
9.3.3.2 PLATFORM BASED PRODUCTS
9.3.3.3 KITS AND REAGENTS
9.3.3.4 OTHER CONSUMABLES
9.3.4 LYMPHOPLASMACYTIC LYMPHOMA
9.3.4.1 INSTRUMENT BASED PRODUCTS
9.3.4.2 PLATFORM BASED PRODUCTS
9.3.4.3 KITS AND REAGENTS
9.3.4.4 OTHER CONSUMABLES
9.3.5 SMALL-CELL LYMPHOCYTIC LYMPHOMA
9.3.5.1 INSTRUMENT BASED PRODUCTS
9.3.5.2 PLATFORM BASED PRODUCTS
9.3.5.3 KITS AND REAGENTS
9.3.5.4 OTHER CONSUMABLES
10 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY APPLICATION
10.1 OVERVIEW
10.2 SCREENING
10.2.1 INSTRUMENT BASED PRODUCTS
10.2.2 PLATFORM BASED PRODUCTS
10.2.3 KITS AND REAGENTS
10.2.4 OTHER CONSUMABLES
10.3 DIAGNOSTIC AND PREDICTIVE
10.3.1 INSTRUMENT BASED PRODUCTS
10.3.2 PLATFORM BASED PRODUCTS
10.3.3 KITS AND REAGENTS
10.3.4 OTHER CONSUMABLES
10.4 PROGNOSTIC
10.4.1 INSTRUMENT BASED PRODUCTS
10.4.2 PLATFORM BASED PRODUCTS
10.4.3 KITS AND REAGENTS
10.4.4 OTHER CONSUMABLES
10.5 RESEARCH
10.5.1 INSTRUMENT BASED PRODUCTS
10.5.2 PLATFORM BASED PRODUCTS
10.5.3 KITS AND REAGENTS
10.5.4 OTHER CONSUMABLES
11 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE
11.1 OVERVIEW
11.2 STAGE IV
11.3 STAGE I
11.4 STAGE III
11.5 STAGE II
11.6 STAGE 0
12 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY TECHNOLOGY
12.1 OVERVIEW
12.2 FLUORESCENT IN SITU HYBRIDIZATION
12.3 NEXT GENERATION SEQUENCING
12.4 FLUORIMMUNOASSAY
12.5 COMPARATIVE GENOMIC HYBRIDIZATION
12.6 IMMUNOHISTOLOCHEMICAL
12.7 OTHER
13 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY PRODUCT
13.1 OVERVIEW
13.2 INSTRUMENT BASED PRODUCTS
13.2.1 IMAGING
13.2.2 BIOPSY
13.3 PLATFORM BASED PRODUCTS
13.3.1 NEXT-GENERATION SEQUENCING
13.3.2 MICROARRAYS
13.3.3 PCR
13.3.4 OTHERS
13.4 KITS AND REAGENTS
13.4.1 NON-HODGKIN LYMPHOMA PANELS
13.4.2 IMMUNOHISTOCHEMISTRY STAINS
13.4.3 OTHERS
13.5 OTHER CONSUMABLES
14 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 DIAGNOSTIC CENTERS
14.4 CANCER RESEARCH CENTERS
14.5 ACADEMIC INSTITUTES
14.6 AMBULATORY SURGICAL CENTERS
14.7 OTHERS
15 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
15.4 OTHERS
16 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION
16.1 MIDDLE EAST AND AFRICA
16.1.1 SOUTH AFRICA
16.1.2 SAUDI ARABIA
16.1.3 U.A.E.
16.1.4 EGYPT
16.1.5 ISRAEL
16.1.6 REST OF MIDDLE EAST AND AFRICA
17 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
18 SWOT ANALYSIS
19 MIDDLE EAST & AFRICA NON-HODKIN LYMPHOMA DIAGNOSTICS MARKET
19.1 CANON MEDICAL SYSTEMS CORPORATION
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 KONINKLIJKE PHILIPS N.V.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 SIEMENS HEALTHCARE GMBH
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENT
19.4 DANAHER.
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENTS
19.5 BIO-RAD LABORATORIES, INC.
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.6 AGILENT TECHNOLOGIES, INC.
19.6.1 COMPANY PROFILE
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENT
19.7 CENTOGENE N.V.
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 F. HOFFMANN- LA ROCHE LTD
19.8.1 COMPANY SNAPSHOT
19.8.2 REVENUE ANALYSIS
19.8.3 PRODUCT PORTFOLIO
19.8.4 RECENT DEVELOPMENTS
19.9 GENERAL ELECTRIC COMPANY
19.9.1 COMPANY SNAPSHOT
19.9.2 REVENUE ANALYSIS
19.9.3 PRODUCT PORTFOLIO
19.9.4 RECENT DEVELOPMENTS
19.1 GENEDX, LLC
19.10.1 COMPANY SNAPSHOT
19.10.2 PRODUCT PORTFOLIO
19.10.3 RECENT DEVELOPMENT
19.11 GRAIL
19.11.1 COMPANY PROFILE
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 HOLOGIC INC.
19.12.1 COMPANY SNAPSHOT
19.12.2 REVENUE ANALYSIS
19.12.3 PRODUCT PORTFOLIO
19.12.4 RECENT DEVELOPMENT
19.13 INVITAE CORPORATION
19.13.1 COMPANY PROFILE
19.13.2 REVENUE ANALYSIS
19.13.3 PRODUCT PORTFOLIO
19.13.4 RECENT DEVELOPMENT
19.14 NEUSOFT CORPORATION
19.14.1 COMPANY SNAPSHOT
19.14.2 REVENUE ANALYSIS
19.14.3 PRODUCT PORTFOLIO
19.14.4 RECENT DEVELOPMENT
19.15 NEOGENOMICS LABORATORIES
19.15.1 COMPANY SNAPSHOT
19.15.2 REVENUE ANALYSIS
19.15.3 PRODUCT PORTFOLIO
19.15.4 RECENT DEVELOPMENTS
19.16 PERKINELMER INC
19.16.1 COMPANY PROFILE
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.17 QIAGEN
19.17.1 COMPANY SNAPSHOT
19.17.2 REVENUE ANALYSIS
19.17.3 PRODUCT PORTFOLIO
19.17.4 RECENT DEVELOPMENT
19.18 SYSMEX CORPORATION
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENTS
20 QUESTIONNAIRE
21 RELATED REPORTS
Lista de Tablas
TABLE 1 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA IMMUNOHISTOCHEMISTRY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA GENETIC TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA CYTOGENETICS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA LUMBAR PUNCTURE (SPINAL TAP) IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA BLOOD TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA CYTOCHEMISTRY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA OTHERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA LYMPHOPLASMACYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA STAGE IV IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA STAGE I IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA STAGE III IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA STAGE II IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION
TABLE 45 MIDDLE EAST & AFRICA STAGE 0 IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA FLUORESCENT IN SITU HYBRIDIZATION IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA NEXT GENERATION SEQUENCING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA FLUORIMMUNOASSAY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA COMPARATIVE GENOMIC HYBRIDIZATION IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA IMMUNOHISTOCHEMICAL IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA OTHERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 MIDDLE EAST & AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 55 MIDDLE EAST & AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 56 MIDDLE EAST & AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 57 MIDDLE EAST & AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 58 MIDDLE EAST & AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 MIDDLE EAST & AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 60 MIDDLE EAST & AFRICA OTHER CONSUMABLES IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION))
TABLE 61 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 62 MIDDLE EAST & AFRICA HOSPITALS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 MIDDLE EAST & AFRICA DIAGNOSTIC CENTERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 MIDDLE EAST & AFRICA CANCER RESEARCH CENTERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 65 MIDDLE EAST & AFRICA ACADEMIC INSTITUTES IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 66 MIDDLE EAST & AFRICA AMBULATORY SURGICAL CENTERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 67 MIDDLE EAST & AFRICA OTHERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 68 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 69 MIDDLE EAST & AFRICA DIRECT TENDER IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 70 MIDDLE EAST & AFRICA RETAIL SALES IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 71 MIDDLE EAST & AFRICA OTHERS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 72 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 73 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 MIDDLE EAST AND AFRICA IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 75 MIDDLE EAST AND AFRICA BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 76 MIDDLE EAST AND AFRICA BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 77 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 78 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 79 MIDDLE EAST AND AFRICA AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 80 MIDDLE EAST AND AFRICA DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 MIDDLE EAST AND AFRICA ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 MIDDLE EAST AND AFRICA MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 83 MIDDLE EAST AND AFRICA PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 84 MIDDLE EAST AND AFRICA LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 MIDDLE EAST AND AFRICA BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 86 MIDDLE EAST AND AFRICA INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 87 MIDDLE EAST AND AFRICA FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 MIDDLE EAST AND AFRICA CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 MIDDLE EAST AND AFRICA MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 MIDDLE EAST AND AFRICA SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 91 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 92 MIDDLE EAST AND AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 MIDDLE EAST AND AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 94 MIDDLE EAST AND AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 95 MIDDLE EAST AND AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 MIDDLE EAST AND AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 97 MIDDLE EAST AND AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 98 MIDDLE EAST AND AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 99 MIDDLE EAST AND AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 100 MIDDLE EAST AND AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 101 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 102 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 103 MIDDLE EAST AND AFRICA SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 104 MIDDLE EAST AND AFRICA DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 105 MIDDLE EAST AND AFRICA PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 106 MIDDLE EAST AND AFRICA RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 107 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 108 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 109 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 110 SOUTH AFRICA IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 111 SOUTH AFRICA BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 112 SOUTH AFRICA BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 113 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 114 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 115 SOUTH AFRICA AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 116 SOUTH AFRICA DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 SOUTH AFRICA ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 118 SOUTH AFRICA MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 119 SOUTH AFRICA PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 120 SOUTH AFRICA LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 121 SOUTH AFRICA BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 SOUTH AFRICA INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 123 SOUTH AFRICA FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 124 SOUTH AFRICA CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 SOUTH AFRICA MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 126 SOUTH AFRICA LYMPHOPLASMACYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 127 SOUTH AFRICA SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 128 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 129 SOUTH AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 130 SOUTH AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 131 SOUTH AFRICA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 132 SOUTH AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 133 SOUTH AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 134 SOUTH AFRICA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 135 SOUTH AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 136 SOUTH AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 137 SOUTH AFRICA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 138 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 139 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 140 SOUTH AFRICA PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 141 SOUTH AFRICA RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 142 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 143 SOUTH AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 144 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 145 SAUDI ARABIA IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 146 SAUDI ARABIA BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 147 SAUDI ARABIA BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 148 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 149 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 150 SAUDI ARABIA AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 151 SAUDI ARABIA DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 SAUDI ARABIA ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 SAUDI ARABIA MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 SAUDI ARABIA PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 155 SAUDI ARABIA LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 156 SAUDI ARABIA BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 157 SAUDI ARABIA INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 158 SAUDI ARABIA FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 159 SAUDI ARABIA CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 160 SAUDI ARABIA MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 161 SAUDI ARABIA LYMPHOPLASMACYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 162 SAUDI ARABIA SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 163 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 164 SAUDI ARABIA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 165 SAUDI ARABIA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 166 SAUDI ARABIA INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 167 SAUDI ARABIA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 168 SAUDI ARABIA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 169 SAUDI ARABIA PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 170 SAUDI ARABIA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 171 SAUDI ARABIA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 172 SAUDI ARABIA KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 173 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 174 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 175 SAUDI ARABIA SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 176 SAUDI ARABIA DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 177 SAUDI ARABIA PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 178 SAUDI ARABIA RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 179 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 180 SAUDI ARABIA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 181 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 182 U.A.E IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 183 U.A.E BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 184 U.A.E BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 185 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 186 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 187 U.A.E AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 188 U.A.E DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 189 U.A.E ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 190 U.A.E MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 191 U.A.E PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 192 U.A.E LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 193 U.A.E BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 194 U.A.E INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 195 U.A.E FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 196 U.A.E CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 197 U.A.E MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 198 U.A.E LYMPHOPLASMACYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 199 U.A.E SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 200 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 201 U.A.E INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 202 U.A.E INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 203 U.A.E INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 204 U.A.E PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 205 U.A.E PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 206 U.A.E PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 207 U.A.E KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 208 U.A.E KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 209 U.A.E KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 210 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 211 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 212 U.A.E SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 213 U.A.E DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 214 U.A.E PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 215 U.A.E RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 216 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 217 U.A.E NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 218 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 219 EGYPT IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 220 EGYPT BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 221 EGYPT BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 222 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 223 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 224 EGYPT AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 225 EGYPT DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 226 EGYPT ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 227 EGYPT MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 228 EGYPT PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 229 EGYPT LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 230 EGYPT BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 231 EGYPT INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 232 EGYPT FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 233 EGYPT CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 234 EGYPT MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 235 EGYPT LYMPHOPLASMACYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 236 EGYPT SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 237 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 238 EGYPT INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 239 EGYPT INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 240 EGYPT INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 241 EGYPT PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 242 EGYPT PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 243 EGYPT PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 244 EGYPT KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 245 EGYPT KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 246 EGYPT KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 247 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 248 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 249 EGYPT SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 250 EGYPT DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 251 EGYPT PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 252 EGYPT RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 253 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 254 EGYPT NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 255 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 256 ISRAEL IMAGING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 257 ISRAEL BIOPSY IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 258 ISRAEL BIOMARKER TEST IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 259 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 260 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 261 ISRAEL AGGRESSIVE LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 262 ISRAEL DIFFUSE LARGE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 263 ISRAEL ANAPLASTIC LARGE-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 264 ISRAEL MANTLE CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 265 ISRAEL PERIPHERAL T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 266 ISRAEL LYMPHOBLASTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 267 ISRAEL BURKITT LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 268 ISRAEL INDOLENT LYMPHOMAS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 269 ISRAEL FOLLICULAR LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 270 ISRAEL CUTANEOUS T-CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 271 ISRAEL MARGINAL ZONE B CELL LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 272 ISRAEL LYMPHOPLASMACYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 273 ISRAEL SMALL-CELL LYMPHOCYTIC LYMPHOMA IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 274 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 275 ISRAEL INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 276 ISRAEL INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 277 ISRAEL INSTRUMENT BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 278 ISRAEL PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 279 ISRAEL PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 280 ISRAEL PLATFORM BASED PRODUCTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 281 ISRAEL KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 282 ISRAEL KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 283 ISRAEL KITS AND REAGENTS IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 284 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 285 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 286 ISRAEL SCREENING IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 287 ISRAEL DIAGNOSTIC AND PREDICTIVE IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 288 ISRAEL PROGNOSTIC IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 289 ISRAEL RESEARCH IN NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 290 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 291 ISRAEL NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 292 REST OF MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 10 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 12 THE INCREASING PREVALENCE OF NON-HODGKIN LYMPHOMA AND INCREASED RESEARCH & DEVELOPMENT IN NON-HODGKIN LYMPHOMA DIAGNOSTICS ARE DRIVING THE MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 13 REAGENTS & CONSUMABLES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET IN 2021 & 2028
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET
FIGURE 15 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TEST TYPE, 2022
FIGURE 16 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 17 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 18 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 19 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TUMOR TYPE, 2022
FIGURE 20 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 21 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 22 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TUMOR TYPE, LIFELINE CURVE
FIGURE 23 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY APPLICATION, 2022
FIGURE 24 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 25 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 26 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 27 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY CANCER STAGE, 2022
FIGURE 28 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY CANCER STAGE, 2023-2030 (USD MILLION)
FIGURE 29 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY CANCER STAGE, CAGR (2023-2030)
FIGURE 30 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY CANCER STAGE, LIFELINE CURVE
FIGURE 31 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TECHNOLOGY, 2022
FIGURE 32 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 33 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 34 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 35 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY PRODUCT, 2022
FIGURE 36 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 37 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 38 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 39 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY END USER, 2022
FIGURE 40 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 41 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY END USER, CAGR (2023-2030)
FIGURE 42 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 43 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 44 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 45 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 46 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 47 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 48 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 49 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 50 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 51 MIDDLE EAST AND AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 52 MIDDLE EAST & AFRICA NON-HODGKIN LYMPHOMA DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.