Asia Pacific Stroke Market
Taille du marché en milliards USD
TCAC :
% 
 USD
7.01 Billion
USD
13.71 Billion
2024
2032
USD
7.01 Billion
USD
13.71 Billion
2024
2032
| 2025 –2032 | |
| USD 7.01 Billion | |
| USD 13.71 Billion | |
|
|
|
|
Segmentation du marché des accidents vasculaires cérébraux (AVC) en Asie-Pacifique, type (AVC ischémique, accident ischémique transitoire (AIT) et AVC hémorragique ), diagnostic et traitement (diagnostic et traitement), sexe (femme et homme), utilisateur final (hôpitaux et cliniques, cliniques spécialisées, centres de chirurgie ambulatoire, soins à domicile, laboratoires et autres), canal de distribution (direct, vente au détail et en ligne) – Tendances et prévisions du secteur jusqu'en 2032
Analyse du marché des accidents vasculaires cérébraux
Le marché de l'AVC en Asie-Pacifique est un secteur de la santé en pleine croissance, axé sur les produits et services visant à prévenir, diagnostiquer, traiter et rééduquer les patients victimes d'AVC. Les principaux composants de ce marché comprennent les produits pharmaceutiques (tels que les thrombolytiques, les antiplaquettaires et les anticoagulants), les dispositifs médicaux (tels que les stents vasculaires et les dispositifs neuroprotecteurs) et les équipements de rééducation (notamment les outils de physiothérapie et les aides orthophoniques). Cette croissance est tirée par des facteurs tels que la sensibilisation croissante à la prévention des AVC, les avancées technologiques médicales et l'augmentation de la population âgée, plus vulnérable aux AVC. La prévalence croissante des maladies non transmissibles, notamment l'hypertension et le diabète, contribue également à la demande de soins efficaces pour les AVC.
Taille du marché des accidents vasculaires cérébraux
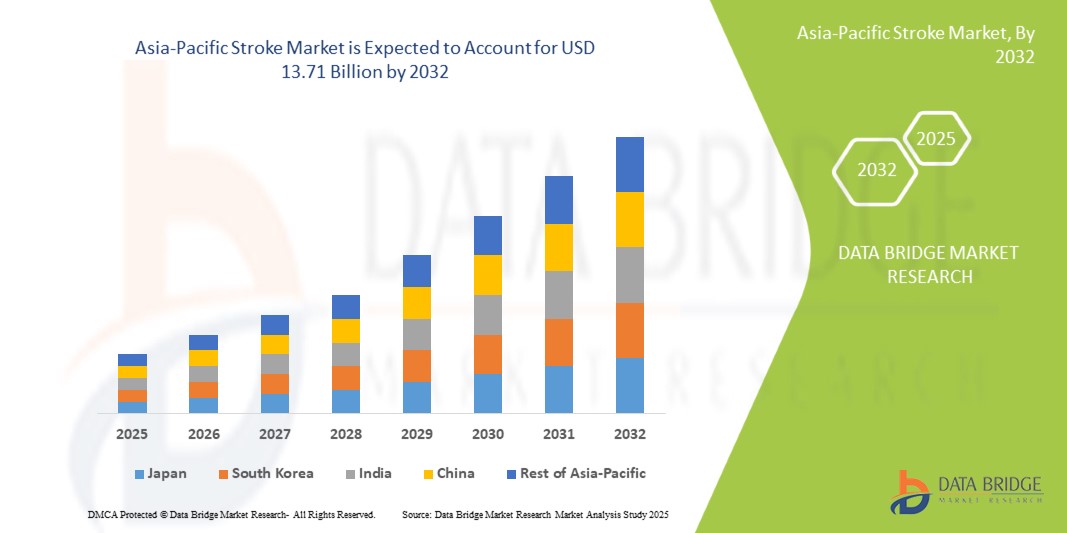
Le marché des accidents vasculaires cérébraux en Asie-Pacifique devrait atteindre 13,71 milliards USD d'ici 2032, contre 7,01 milliards USD en 2024, avec un TCAC de 9,1 % au cours de la période de prévision de 2025 à 2032. Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie par des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
Tendances du marché des accidents vasculaires cérébraux
« Adoption croissante de technologies avancées de neuroimagerie et de solutions de télémédecine pour le diagnostic et la prise en charge des accidents vasculaires cérébraux »
Une tendance notable sur le marché de l'AVC en Asie-Pacifique est l'adoption croissante de technologies avancées de neuroimagerie et de solutions de télémédecine pour le diagnostic et la prise en charge de l'AVC. La prise de conscience croissante de l'importance cruciale d'une intervention rapide dans le traitement de l'AVC entraîne une évolution significative vers l'utilisation de techniques d'imagerie sophistiquées, telles que l'IRM et le scanner, qui permettent un diagnostic plus rapide et plus précis des différents types d'AVC. De plus, la télémédecine devient essentielle pour fournir des consultations et un suivi à distance rapides, notamment dans les zones rurales ou mal desservies, permettant aux professionnels de santé d'évaluer les patients et de mettre en place rapidement les traitements. Cette tendance améliore non seulement les résultats pour les patients, mais stimule également l'innovation et l'investissement dans le continuum de soins de l'AVC.
Portée du rapport et segmentation du marché des accidents vasculaires cérébraux
|
Attributs |
Informations clés sur le marché des accidents vasculaires cérébraux |
|
Segments couverts |
|
|
Pays couverts |
Chine, Japon, Inde, Corée du Sud, Singapour, Malaisie, Australie, Thaïlande, Indonésie, Philippines, Vietnam, Taïwan et reste de l'Asie-Pacifique |
|
Principaux acteurs du marché |
Bristol-Myers Squibb Company (États-Unis), Boehringer Ingelheim International GmbH (Allemagne), F. Hoffmann-La Roche Ltd (Suisse), DAIICHI SANKYO COMPANY, LIMITED (Japon), Sanofi (France), Johnson & Johnson Services, Inc. (États-Unis), Bayer AG (Allemagne), Sandoz AG (Suisse), Pfizer Inc. (États-Unis), Medtronic (Irlande), Abbott (États-Unis), Viatris Inc. (États-Unis), AstraZeneca (Royaume-Uni), Penumbra, Inc. (États-Unis), GLENMARK PHARMACEUTICALS LTD (Inde), Fresenius SE & Co. KGaA (Allemagne), Teva Pharmaceuticals USA, Inc. (Israël), Lupin (Inde) et Amneal Pharmaceuticals LLC (États-Unis), entre autres |
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research incluent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire. |
Définition du marché des accidents vasculaires cérébraux
Le marché de l'AVC en Asie-Pacifique englobe les différents produits, services et technologies impliqués dans la prévention, le diagnostic, le traitement et la réadaptation des patients victimes d'AVC dans le monde entier. Il comprend une gamme de dispositifs médicaux, de produits pharmaceutiques, d'équipements d'imagerie et de solutions thérapeutiques visant à répondre aux complexités de la prise en charge de l'AVC. Ce marché est stimulé par l'incidence croissante des AVC due au vieillissement de la population, aux facteurs liés au mode de vie et à une meilleure connaissance des symptômes et des options thérapeutiques. De plus, les progrès des technologies de santé et de la télémédecine façonnent le paysage, facilitant une meilleure prise en charge des patients et améliorant les résultats de la prise en charge de l'AVC dans divers groupes démographiques et contextes de soins.
Dynamique du marché des accidents vasculaires cérébraux
Conducteurs
- L'augmentation de l'incidence des accidents vasculaires cérébraux (AVC) entraîne une demande accrue de traitements.
L'incidence croissante des accidents vasculaires cérébraux (AVC) est un facteur déterminant pour le marché de l'AVC, influençant à la fois la demande de traitement et les infrastructures de santé. L'AVC, principale cause d'invalidité et de décès dans le monde, est de plus en plus fréquent en raison de divers facteurs de risque, notamment le vieillissement de la population, la sédentarité, l'hypertension artérielle, le diabète, le tabagisme et une mauvaise alimentation. Avec l'allongement de l'espérance de vie et le vieillissement de la population, la prévalence des pathologies contribuant aux AVC, telles que l'hypertension et la fibrillation auriculaire, a également augmenté, ce qui entraîne une augmentation du nombre de personnes victimes d'AVC nécessitant une prise en charge médicale immédiate et une rééducation à long terme.
Par exemple,
En mai 2023, selon un article publié dans eClinicalMedicine, l'AVC était la deuxième cause de décès et la troisième cause d'invalidité dans le monde. Au cours des 30 dernières années, on a constaté une augmentation du nombre absolu d'AVC incidents (70 %) et de prévalence (85 %), ainsi que des décès (43 %) dus à un AVC.
- Augmentation du nombre de patients souffrant d'hypertension et de maladies coronariennes
L'hypertension artérielle, communément appelée hypertension artérielle, est une affection caractérisée par une force accrue du sang contre les parois des artères. Elle se définit généralement par une pression artérielle de 130/80 mm Hg ou plus et peut être qualifiée d'essentielle (primaire) ou secondaire, selon sa cause sous-jacente. Une hypertension prolongée peut entraîner divers problèmes de santé graves, dont l'un des plus importants est la maladie coronarienne. Cette maladie résulte de l'accumulation progressive de dépôts graisseux (athérosclérose) dans les artères coronaires, qui alimentent le muscle cardiaque en oxygène et en nutriments. Lorsque ces artères se rétrécissent ou se bouchent, le flux sanguin vers le cœur est réduit, ce qui entraîne des douleurs thoraciques (angine de poitrine) et, dans les cas graves, des crises cardiaques.
Par exemple,
En septembre 2023, selon un article publié dans le Journal de l'Organisation panaméricaine de la santé, l'hypertension, souvent asymptomatique, contribue significativement aux maladies cardiovasculaires, première cause de décès. Des facteurs tels que le vieillissement, l'obésité et de mauvaises habitudes de vie alimentent cette prévalence croissante, exigeant des traitements efficaces.
Opportunités
- Développement de thérapies avancées pour les accidents vasculaires cérébraux
The development of advanced therapeutics presents a significant opportunity for the stroke market by addressing the substantial unmet needs in stroke care. Current treatments, primarily focused on restoring blood flow through thrombolysis or thrombectomy, are effective only within a narrow time window and do not address the underlying neuronal damage. Advanced therapeutics, such as neuroprotective agents, cell-based therapies, and targeted drug delivery systems, promise to mitigate this damage, promote neuronal repair, and improve long-term functional outcomes for stroke patients. This will lead to a decrease in disability, reduced healthcare costs associated with long-term care, and improved quality of life for survivors, thus expanding the market potential by attracting investment and driving demand for more effective treatments.
For instance,
In April 2022, according to an article published by the American Heart Association Journals, the treatment of acute ischemic stroke continues to advance. Tenecteplase has been evaluated as an alternative thrombolytic drug and evidence suggests that it is as least as effective as alteplase and may lyse large vessel clots more effectively. Endovascular therapy with mechanical thrombectomy has now been shown to be beneficial up to 24 hours after stroke onset in carefully selected patients with proximal, large vessel occlusions.
- Expansion in Stroke Rehabilitation Services
The expansion of stroke rehabilitation services presents a substantial opportunity for the stroke market by addressing the growing need for more effective recovery and rehabilitation programs. Currently, stroke survivors often face significant challenges in regaining lost motor and cognitive functions, leading to prolonged hospital stays, increased medical costs, and reduced quality of life. As the global population ages and stroke incidence rates rise, there is a pressing need for enhanced rehabilitation services that cater to the individual needs of stroke survivors. By expanding stroke rehabilitation services, healthcare providers and payers can address the unmet demand for comprehensive and personalized care, leading to better patient outcomes, reduced healthcare costs, and increased patient satisfaction.
For instance,
In April 2023, according to an article published by the MDPI, developed countries make efforts to provide rehabilitation for stroke patients. Physical rehabilitation can reduce or prevent known complications in stroke patients while also improving their quality of life. Therapists choose interventions based on impairments, activity limitations, and goals of recovery.
Restraints/Challenges
- High Cost of Diagnosis
Heart disease and stroke are one of the major factors of increasing mortality rate globally over the years. Stroke can be ranked among the most costly chronic diseases. More than 868,000 Americans die due to heart disease or stroke every year—that’s one-third of all deaths. With the increasing incidence of stroke, the cost of diagnosis and treatment has increased over the years which is the major restraining factor.
The majority of patients not only endure life-long disabilities that affect their livelihood but also have an enormous societal economic impact. The cost of diagnosis has also increased with the rise in technological advancement.
For instance,
According to the Agency for Healthcare Research and Quality, the average hospital admission for ischemic stroke (which includes diagnosis & stay) is 5.6 days at USD 9,100 per stay, and for hemorrhagic stroke, it is 8.4 days at USD 19,500 per stay.
- Increase in Product Recall
A wide range of diagnostic devices for stroke are used by professionals for performing different procedures on various patients of different age groups. Thus, side effects and complications associated with the use of these devices can cause serious harm to the patients.
Also these diagnostic devices and products are very expensive and highly risky for which a potential failure may cause serious consequences to the patient. Therefore, they are strictly regulated and recalled for the safety of patients.
For instance,
Neusoft Medical Systems Co., Ltd. Company’s product NeuViz 64 Multi-slice CT Scanner System Product which is a Multi-Slice CT Scanner System used as a whole body computed tomography x-ray system featuring a continuously rotating x-ray tube and detector array have been recalled by FDA due to the software error into the system.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Stroke Market Scope
The market is categorized into five notable segments based on type, diagnosis & treatment, gender, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Ischemic Stroke
- Thrombotic (Cerebral Thrombosis)
- Embolic (Cerebral Embolism)
- Hemorrhagic Stroke
- Subarachnoid Hemorrhage
- Hémorragie intracérébrale
- Accident ischémique transitoire (AIT)
Diagnostic et traitement
- Traitement
- Médicament
- Par classe
- Médicaments contre la tension artérielle
- Inhibiteurs de l'enzyme de conversion de l'angiotensine (ECA)
- Ramipril
- Lisinopril
- Énalapril
- Périndopril
- Autre
- diurétiques thiazidiques
- Indapamide
- Bendrofluméthiazide
- Spironolactone
- Amiloride
- Autre
- Inhibiteurs des canaux calciques
- Amlodipine
- Nifédipine
- Vérapamil
- Nicardipine
- Félodipine
- Nimodipine
- Autre
- Bêta-bloquants
- Aténolol
- Bisoprolol
- Labétolol
- Autres
- Alpha-bloquants
- Doxazosine
- Autres
- Autres
- Inhibiteurs de l'enzyme de conversion de l'angiotensine (ECA)
- médicaments antiplaquettaires
- Aspirine
- Clopidogrel
- Dipyridamole
- Ticlopidine
- Autres
- Anticoagulants
- Warfarine
- Apixaban
- Dabigatran
- Héparine
- Rivaroxaban
- Autre
- Activateur tissulaire du plasminogène (TPA)
- Alteplase
- Ténéctéplase
- Rétéplase
- Anistréplase
- Autre
- Statines
- Atorvastatine
- Simvastatine
- Lovastatine
- Rosuvastatine
- Fluvastatine
- Pravastatine
- Pitavastatine
- Autres
- Vitamine K
- Médicaments de soutien
- Compléments alimentaires
- Antipyrétiques
- Autres
- Médicaments contre la tension artérielle
- Par type de médicament
- De marque
- Activase
- Édobaxan
- Coumadin
- Héparine Léo
- Duoplavine
- Aggrenox
- Rétavase
- Jantoven
- Cathflo
- Autre
- De marque
- Générique
- Par voie d'administration
- Oral
- Comprimé
- Capsules
- Autres
- Parentérale
- Intraveineux
- Sous-cutané
- Autres
- Oral
- Par mode d'achat
- Ordonnance
- En vente libre (OTC)
- Par type de thérapie
- Thérapie combinée
- Monothérapie
- Par voie d'administration
- Chirurgie
- Bobines emboliques
- Cathéters d'aspiration
- Récupérateur de stent
- Coupe chirurgicale
- Autres
- Thérapie des autres
- physiothérapie
- Ergothérapie
- orthophonie
- Autres
- Par classe
- Diagnostic
- Test d'imagerie
- Tomodensitométrie (TDM)
- Imagerie par résonance magnétique (IRM)
- Échographie carotidienne
- Angiographie cérébrale
- Analyse de sang
- Échocardiogramme
- Ponction lombaire
- Autres
- Test d'imagerie
- Médicament
Genre
- Femelle
- Mâle
Utilisateur final
- Hôpitaux et cliniques
- Cliniques spécialisées
- Centre de chirurgie ambulatoire
- Soins à domicile
- Laboratoires
- Autres
Canal de distribution
- Direct
- Vente au détail
- En ligne
Analyse régionale du marché des accidents vasculaires cérébraux
Le marché est analysé et des informations sur la taille du marché et les tendances sont fournies par type, diagnostic et traitement, sexe, utilisateur final et canal de distribution comme référencé ci-dessus.
Les pays couverts par le marché sont la Chine, le Japon, l'Inde, la Corée du Sud, Singapour, la Malaisie, l'Australie, la Thaïlande, l'Indonésie, les Philippines, le Vietnam, Taïwan et le reste de l'Asie-Pacifique.
La Chine devrait dominer et connaître la croissance la plus rapide sur le marché des accidents vasculaires cérébraux en Asie-Pacifique en raison de ses dépenses de santé élevées, de son infrastructure médicale avancée et de ses programmes complets de soins des accidents vasculaires cérébraux.
La section pays du rapport présente également les facteurs d'impact sur les marchés individuels et les évolutions réglementaires nationales qui influencent les tendances actuelles et futures du marché. Des données telles que l'analyse des chaînes de valeur en aval et en amont, les tendances techniques, l'analyse des cinq forces de Porter et les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario de marché pour chaque pays. De plus, la présence et la disponibilité des marques d'Asie-Pacifique et les difficultés auxquelles elles sont confrontées en raison de la forte ou de la faible concurrence des marques locales et nationales, de l'impact des tarifs douaniers nationaux et des routes commerciales sont prises en compte lors de l'analyse prévisionnelle des données nationales.
Part de marché des accidents vasculaires cérébraux
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Stroke Market Leaders Operating in the Market Are:
- Bristol-Myers Squibb Company (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Sanofi (France)
- Johnson & Johnson Services, Inc. (U.S.)
- Bayer AG (Germany)
- Sandoz AG (Switzerland)
- Pfizer Inc. (U.S.)
- Medtronic (Ireland)
- Abbott (U.S.)
- Viatris Inc. (U.S.)
- AstraZeneca (U.K.)
- Penumbra, Inc. (U.S.)
- GLENMARK PHARMACEUTICALS LTD (India)
- Fresenius SE & Co. KGaA (Germany)
- Teva Pharmaceuticals USA, Inc. (Israel)
- Lupin (India)
- Amneal Pharmaceuticals LLC (U.S.)
Latest Developments in Stroke Market
- In July 2023, Roche announced a new partnership with Alnylam to develop and commercialize zilebesir, an investigational RNAi therapy currently in Phase 2 for the treatment of high blood pressure. This collaboration combines Alnylam's proven experience in RNAi therapy with Roche's global commercial reach, commitment to innovation and desire to change the landscape for patients with serious cardiovascular disease
- In September 2020, Daiichi Sankyo Company Limited announced that it has submitted a supplemental application in Japan for the extended approval of the anticoagulant edoxaban (edoxaban benzoate hydrate) in elderly patients with nonvalvular regurgitation and severe bleeding. Risk. This application is based on the results of a Japanese Phase 3 clinical trial (ELDERCARE-AF trial) in 984 patients with non-valvular atrial fibrillation who are at least 80 years old and have a high risk of bleeding and are not suitable for other available anticoagulant therapies. Daiichi Sankyo plans to contribute to the treatment of elderly patients with non-valvular atrial fibrillation by offering a new treatment option
- In July 2022, Sandoz, the world's leading manufacturer of generics and biosimilars, announced an investment of approximately USD 90 million in its facility in Ljubljana, Slovenia, to establish its Sandoz Biopharma Development Center by 2026. With this investment, the Ljubljana site will become one of Sandoz's most important biosimilar product development sites. The new office will result in the creation of approximately 200 new full-time jobs and will further strengthen the company's capabilities in the development of biosimilars and pharmaceutical products
- In January 2023, Penumbra, Inc., a global healthcare company focused on innovative therapies, announced the US Food and Drug Administration (FDA) approval and launch of Lightning Flash™, the most advanced and powerful mechanical thrombectomy system on the market. Lightning Flash features Penumbra's new Lightning Intelligent Aspiration technology, which now has two clot detection algorithms. Combined with innovative catheter technology, the Lightning Flash is designed to quickly remove large blood clots from the body, including venous embolism and pulmonary embolism (PE). This launch will help the company expand its product portfolio because the advanced results of this new technology are exceptionally traceable and its unique ability to differentiate flowing blood from clots
- In August 2023, Lupin has announced the launch of Jeet, a patient support program dedicated to heart health. The launch of the initiative coincides with India's 77th Independence Day, which symbolizes freedom from disease-related stress and the journey to a happier and healthier life. Jeet becomes a trusted partner in cardiovascular care by offering various benefits such as cost savings, medical assistance, medication reminders and lifestyle support. Jeet offers a holistic approach to improving the physician and patient experience by increasing awareness of cardiovascular disease and its comorbidities. The app includes features designed to encourage a healthier lifestyle and support a healthy heart
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 LIMITATIONS
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 ASIA-PACIFIC STROKE MARKET, REGULATORY FRAMEWORK
5.1 REGULATION IN U.S.
5.2 REGULATION IN EUROPE
5.3 REGULATION IN CHINA
5.4 REGULATION IN JAPAN
5.5 REGULATION IN SOUTH AFRICA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCES OF STROKE DRIVE DEMAND FOR TREATMENTS
6.1.2 INCREASING NUMBER OF PATIENTS WITH HYPERTENSION AND CORONARY HEART DISEASES
6.1.3 INCREASING DIABETIC AND OBESE POPULATIONS ELEVATE STROKE RISKS
6.1.4 ADVANCEMENTS IN MEDICAL TECHNOLOGY IMPROVE STROKE CARE OUTCO.MES
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSIS
6.2.2 INCREASE IN PRODUCT RECALL
6.3 OPPORTUNITIES
6.3.1 DEVELOPMENT OF ADVANCED THERAPEUTICS FOR STROKES
6.3.2 EXPANSION IN STROKE REHABILITATION SERVICES
6.3.3 INNOVATIVE TREATMENTS IN PIPELINE FOR STROKE TREATMENT
6.4 CHALLENGES
6.4.1 FALSE DIAGNOSIS IN STROKES
6.4.2 COMPLICATIONS ASSOCIATED WITH MANAGING STROKE
7 ASIA-PACIFIC STROKE MARKET, BY TYPE
7.1 OVERVIEW
7.2 ISCHEMIC STROKE
7.2.1 THROMBOTIC (CEREBRAL THROMBOSIS)
7.2.2 EMBOLIC (CEREBRAL EMBOLISM)
7.3 HEMORRHAGIC STROKE
7.3.1 SUBARACHNOID HEMORRHAGE
7.3.2 INTRACEREBRAL HEMORRHAGE
7.4 TRANSIENT ISCHEMIC ATTACT (TIA)
8 ASIA-PACIFIC STROKE MARKET, BY GENDER
8.1 OVERVIEW
8.2 FEMALE
8.3 MALE
9 ASIA-PACIFIC STROKE MARKET, BY DIAGNOSIS AND TREATMENT
9.1 OVERVIEW
9.2 TREATMENT
9.2.1 BY TREATMENT TYPE
9.2.1.1 MEDICATION
9.2.1.1.1 MEDICATION, BY CLASS
9.2.1.1.1.1 BLOOD PRESSURE MEDICINES
9.2.1.1.1.1.1 ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS
9.2.1.1.1.1.2 RAMIPRIL
9.2.1.1.1.1.3 LISINOPRIL
9.2.1.1.1.1.4 ENALAPRIL
9.2.1.1.1.1.5 PERINDOPRIL
9.2.1.1.1.1.6 OTHER
9.2.1.1.1.2 THIAZIDE DIURETICS
9.2.1.1.1.2.1 INDAPAMIDE
9.2.1.1.1.2.2 BENDROFLUMETHIAZIDE
9.2.1.1.1.2.3 SPIRONOLACTONE
9.2.1.1.1.2.4 AMILORIDE
9.2.1.1.1.2.5 OTHER
9.2.1.1.1.3 CALCIUM CHANNEL BLOCKERS
9.2.1.1.1.3.1 AMLODIPINE
9.2.1.1.1.3.2 NIFEDIPINE
9.2.1.1.1.3.3 VERAPAMIL
9.2.1.1.1.3.4 NICARDIPINE
9.2.1.1.1.3.5 FELODIPINE
9.2.1.1.1.3.6 NIMODIPINE
9.2.1.1.1.3.7 OTHERS
9.2.1.1.1.4 BETA BLOCKERS
9.2.1.1.1.4.1 ATENOLOL
9.2.1.1.1.4.2 BISOPROLOL
9.2.1.1.1.4.3 LABETOLOL
9.2.1.1.1.4.4 OTHERS
9.2.1.1.1.5 ALPHA-BLOCKERS
9.2.1.1.1.5.1 DOXAZOSIN
9.2.1.1.1.5.2 OTHERS
9.2.1.1.1.6 OTHERS
9.2.1.1.1.7 ANTIPLATELET DRUGS
9.2.1.1.1.7.1 ASPIRIN
9.2.1.1.1.7.2 CLOPIDOGREL
9.2.1.1.1.7.3 DIPYRIDAMOLE
9.2.1.1.1.7.4 TICLOPIDINE
9.2.1.1.1.7.5 OTHERS
9.2.1.1.1.8 ANTICOAGULANTS
9.2.1.1.1.8.1 WARFARIN
9.2.1.1.1.8.2 APIXABAN
9.2.1.1.1.8.3 DABIGATRAN
9.2.1.1.1.8.4 HEPARIN
9.2.1.1.1.8.5 RIVAROXABAN
9.2.1.1.1.8.6 OTHERS
9.2.1.1.1.9 TISSUE PLASMINOGEN ACTIVATOR (TPA)
9.2.1.1.1.9.1 ALTEPLASE
9.2.1.1.1.9.2 TENECTEPLASE
9.2.1.1.1.9.3 RETEPLASE
9.2.1.1.1.9.4 ANISTREPLASE
9.2.1.1.1.9.5 OTHERS
9.2.1.1.1.10 STATINS
9.2.1.1.1.10.1.1 ATORVASTATIN
9.2.1.1.1.10.1.2 SIMVASTATIN
9.2.1.1.1.10.1.3 LOVASTATIN
9.2.1.1.1.10.1.4 ROSUVASTATIN
9.2.1.1.1.10.1.5 FLUVASTATIN
9.2.1.1.1.10.1.6 PRAVASTATIN
9.2.1.1.1.10.1.7 PITAVASTATIN
9.2.1.1.1.10.1.8 OTHERS
9.2.1.1.1.11 VITAMIN K
9.2.1.1.1.12 SUPPORTIVE MEDICATION
9.2.1.1.1.12.1 NUTRITIONAL SUPPLEMENTS
9.2.1.1.1.12.2 ANTIPYRETICS
9.2.1.1.1.12.3 OTHERS
9.2.1.1.2 MEDICATION, BY DRUG TYPE
9.2.1.1.2.1 BRANDED
9.2.1.1.2.1.1 ACTIVASE
9.2.1.1.2.1.2 EDOBAXAN
9.2.1.1.2.1.3 COUMADIN
9.2.1.1.2.1.4 HEPARIN LEO
9.2.1.1.2.1.5 DUOPLAVIN
9.2.1.1.2.1.6 AGGRENOX
9.2.1.1.2.1.7 RETAVASE
9.2.1.1.2.1.8 JANTOVEN
9.2.1.1.2.1.9 CATHFLO
9.2.1.1.2.1.10 OTHER
9.2.1.1.2.2 GENERIC
9.2.1.1.3 MEDICATION, BY ROUTE OF ADMINISTRATION
9.2.1.1.3.1 ORAL
9.2.1.1.3.1.1 TABLET
9.2.1.1.3.1.2 CAPSULES
9.2.1.1.3.1.3 OTHERS
9.2.1.1.3.2 PARENTERAL
9.2.1.1.3.2.1 INTRAVENOUS
9.2.1.1.3.2.2 SUBCUTANEOUS
9.2.1.1.3.3 OTHERS
9.2.1.1.4 MEDICATION, BY MODE OF PURCHASE
9.2.1.1.4.1 PRESCRIPTION
9.2.1.1.4.2 OVER THE COUNTER (OTC)
9.2.1.1.5 MEDICATION, BY THERAPY TYPE
9.2.1.1.5.1 COMBINATION THERAPY
9.2.1.1.5.2 MONOTHERAPY
9.2.1.2 SURGERY
9.2.1.2.1 EMBOLIC COILS
9.2.1.2.2 ASPIRATION CATHETERS
9.2.1.2.3 STENT RETRIEVER
9.2.1.2.4 SURGICAL CLIPPING
9.2.1.2.5 OTHERS
9.2.1.3 OTHERS THERAPY
9.2.1.3.1 PHYSICAL THERAPY
9.2.1.3.2 OCCUPATIONAL THERAPY
9.2.1.3.3 SPEECH THERAPY
9.2.1.3.4 OTHERS
9.3 DIAGNOSIS
9.3.1 IMAGING TEST
9.3.1.1 COMPUTERIZED TOMOGRAPHY (CT) SCAN
9.3.1.2 MAGNETIC RESONANCE IMAGING (MRI)
9.3.1.3 CAROTID ULTRASOUND
9.3.1.4 CEREBRAL ANGIOGRAM
9.3.2 BLOOD TEST
9.3.3 ECHOCARDIOGRAM
9.3.4 LUMBAR PUNCTURE
9.3.5 OTHERS
10 ASIA-PACIFIC STROKE MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT
10.3 RETAIL
10.4 ONLINE
11 ASIA-PACIFIC STROKE MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS & CLINICS
11.3 SPECIALTY CLINICS
11.4 AMBULATORY SURGICAL CENTER
11.5 HOMECARE
11.6 LABORATORIES
11.7 OTHERS
12 ASIA-PACIFIC STROKE MARKET, BY REGION
12.1 ASIA-PACIFIC
12.1.1 CHINA
12.1.2 JAPAN
12.1.3 INDIA
12.1.4 AUSTRALIA
12.1.5 SOUTH KOREA
12.1.6 SINGAPORE
12.1.7 THAILAND
12.1.8 PHILIPPINES
12.1.9 MALAYSIA
12.1.10 INDONESIA
12.1.11 VIETNAM
12.1.12 TAIWAN
12.1.13 REST OF ASIA-PACIFIC
13 ASIA-PACIFIC STROKE MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BRISTOL-MYERS SQUIBB COMPANY
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 DAIICHI SANKYO COMPANY, LIMITED
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 SANOFI
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AMNEAL PHARMACEUTICALS LLC
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 ASTRAZENECA
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 BAYER AG
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENT
15.1 FRESENIUS SE & CO. KGAA
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 GLENMARK PHARMACEUTICALS LTD.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 JOHNSON & JOHNSON SERVICES, INC.
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 LUPIN
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENT
15.14 MEDTRONIC
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 PENUMBRA, INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 REVENUE ANALYSIS
15.15.3 PRODUCT PORTFOLIO
15.15.4 RECENT DEVELOPMENT
15.16 PFIZER INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 SANDOZ AG
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 TEVA PHARMACEUTICALS USA, INC.
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 VIATRIS INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Liste des tableaux
TABLE 1 ASIA-PACIFIC STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 ASIA-PACIFIC ISCHEMIC STROKE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 ASIA-PACIFIC ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 ASIA-PACIFIC HEMORRHAGIC STROKE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 5 ASIA-PACIFIC HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 ASIA-PACIFIC TRANSIENT ISCHEMIC ATTACT (TIA) IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 ASIA-PACIFIC STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 8 ASIA-PACIFIC FEMALE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 ASIA-PACIFIC MALE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 10 ASIA-PACIFIC STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 11 ASIA-PACIFIC TREATMENT IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 ASIA-PACIFIC TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 13 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 14 ASIA-PACIFIC BLOOD PRESSURE MEDICINES IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 15 ASIA-PACIFIC ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 16 ASIA-PACIFIC THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 17 ASIA-PACIFIC CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 18 ASIA-PACIFIC BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 19 ASIA-PACIFIC ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 20 ASIA-PACIFIC ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 21 ASIA-PACIFIC ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 22 ASIA-PACIFIC TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 23 ASIA-PACIFIC STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 24 ASIA-PACIFIC SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 25 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 26 ASIA-PACIFIC BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 27 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 28 ASIA-PACIFIC ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 ASIA-PACIFIC PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 31 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 32 ASIA-PACIFIC SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 ASIA-PACIFIC OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 ASIA-PACIFIC DIAGNOSIS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 35 ASIA-PACIFIC DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 ASIA-PACIFIC IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 ASIA-PACIFIC STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 38 ASIA-PACIFIC DIRECT IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 ASIA-PACIFIC RETAIL IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 40 ASIA-PACIFIC ONLINE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 41 ASIA-PACIFIC STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 42 ASIA-PACIFIC HOSPITALS & CLINICS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 43 ASIA-PACIFIC SPECIALTY CLINICS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 44 ASIA-PACIFIC AMBULATORY SURGICAL CENTER IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 45 ASIA-PACIFIC HOMECARE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 46 ASIA-PACIFIC LABORATORIES IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 47 ASIA-PACIFIC OTHERS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 48 ASIA-PACIFIC STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 49 ASIA-PACIFIC STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 ASIA-PACIFIC ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 ASIA-PACIFIC HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 52 ASIA-PACIFIC STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 53 ASIA-PACIFIC TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 55 ASIA-PACIFIC BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 56 ASIA-PACIFIC ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 57 ASIA-PACIFIC THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 58 ASIA-PACIFIC CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 59 ASIA-PACIFIC BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 60 ASIA-PACIFIC ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 61 ASIA-PACIFIC ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 62 ASIA-PACIFIC ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 63 ASIA-PACIFIC TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 64 ASIA-PACIFIC STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 65 ASIA-PACIFIC SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 66 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 ASIA-PACIFIC BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 69 ASIA-PACIFIC ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 ASIA-PACIFIC PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 71 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 72 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 73 ASIA-PACIFIC SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 ASIA-PACIFIC OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 ASIA-PACIFIC DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 ASIA-PACIFIC IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 ASIA-PACIFIC STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 78 ASIA-PACIFIC STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 79 ASIA-PACIFIC STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 80 CHINA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 CHINA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 CHINA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 CHINA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 84 CHINA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 CHINA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 86 CHINA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 87 CHINA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 88 CHINA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 89 CHINA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 90 CHINA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 91 CHINA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 92 CHINA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 93 CHINA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 94 CHINA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 95 CHINA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 96 CHINA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 97 CHINA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 CHINA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 CHINA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 100 CHINA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 CHINA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 CHINA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 103 CHINA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 104 CHINA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 CHINA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 CHINA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 CHINA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 CHINA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 109 CHINA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 110 CHINA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 111 JAPAN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 JAPAN ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 JAPAN HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 JAPAN STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 115 JAPAN TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 JAPAN MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 117 JAPAN BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 118 JAPAN ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 119 JAPAN THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 120 JAPAN CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 121 JAPAN BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 122 JAPAN ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 123 JAPAN ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 124 JAPAN ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 125 JAPAN TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 126 JAPAN STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 127 JAPAN SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 128 JAPAN MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 JAPAN BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 130 JAPAN MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 131 JAPAN ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 JAPAN PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 JAPAN MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 134 JAPAN MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 JAPAN SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 JAPAN OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 JAPAN DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 JAPAN IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 JAPAN STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 140 JAPAN STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 141 JAPAN STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 142 INDIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 143 INDIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 INDIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 INDIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 146 INDIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 147 INDIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 148 INDIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 149 INDIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 150 INDIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 151 INDIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 152 INDIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 153 INDIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 154 INDIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 155 INDIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 156 INDIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 157 INDIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 158 INDIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 159 INDIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 INDIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 161 INDIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 162 INDIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 INDIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 INDIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 165 INDIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 166 INDIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 INDIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 168 INDIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 INDIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 INDIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 171 INDIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 172 INDIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 173 AUSTRALIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 AUSTRALIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 AUSTRALIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 AUSTRALIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 177 AUSTRALIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 AUSTRALIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 179 AUSTRALIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 180 AUSTRALIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 181 AUSTRALIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 182 AUSTRALIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 183 AUSTRALIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 184 AUSTRALIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 185 AUSTRALIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 186 AUSTRALIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 187 AUSTRALIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 188 AUSTRALIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 189 AUSTRALIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 190 AUSTRALIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 191 AUSTRALIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 AUSTRALIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 193 AUSTRALIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 AUSTRALIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 AUSTRALIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 196 AUSTRALIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 AUSTRALIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 AUSTRALIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 199 AUSTRALIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 200 AUSTRALIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 AUSTRALIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 202 AUSTRALIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 203 AUSTRALIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 204 SOUTH KOREA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 SOUTH KOREA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 206 SOUTH KOREA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 SOUTH KOREA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 208 SOUTH KOREA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 SOUTH KOREA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 210 SOUTH KOREA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 211 SOUTH KOREA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 212 SOUTH KOREA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 213 SOUTH KOREA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 214 SOUTH KOREA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 215 SOUTH KOREA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 216 SOUTH KOREA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 217 SOUTH KOREA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 218 SOUTH KOREA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 219 SOUTH KOREA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 220 SOUTH KOREA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 221 SOUTH KOREA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 SOUTH KOREA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 SOUTH KOREA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 224 SOUTH KOREA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 225 SOUTH KOREA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 SOUTH KOREA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 227 SOUTH KOREA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 SOUTH KOREA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 229 SOUTH KOREA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 SOUTH KOREA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 SOUTH KOREA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 SOUTH KOREA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 233 SOUTH KOREA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 234 SOUTH KOREA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 235 SINGAPORE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 SINGAPORE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 237 SINGAPORE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 238 SINGAPORE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 239 SINGAPORE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 SINGAPORE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 241 SINGAPORE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 242 SINGAPORE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 243 SINGAPORE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 244 SINGAPORE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 245 SINGAPORE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 246 SINGAPORE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 247 SINGAPORE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 248 SINGAPORE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 249 SINGAPORE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 250 SINGAPORE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 251 SINGAPORE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 252 SINGAPORE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 SINGAPORE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 SINGAPORE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 255 SINGAPORE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 256 SINGAPORE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 257 SINGAPORE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 258 SINGAPORE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 SINGAPORE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 SINGAPORE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 261 SINGAPORE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 SINGAPORE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 263 SINGAPORE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 264 SINGAPORE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 265 SINGAPORE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 266 THAILAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 267 THAILAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 THAILAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 THAILAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 270 THAILAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 THAILAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 272 THAILAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 273 THAILAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 274 THAILAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 275 THAILAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 276 THAILAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 277 THAILAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 278 THAILAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 279 THAILAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 280 THAILAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 281 THAILAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 282 THAILAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 283 THAILAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 THAILAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 THAILAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 286 THAILAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 THAILAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 THAILAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 289 THAILAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 THAILAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 THAILAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 THAILAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 THAILAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 THAILAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 295 THAILAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 296 THAILAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 297 PHILIPPINES STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 PHILIPPINES ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 299 PHILIPPINES HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 PHILIPPINES STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 301 PHILIPPINES TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 PHILIPPINES MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 303 PHILIPPINES BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 304 PHILIPPINES ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 305 PHILIPPINES THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 306 PHILIPPINES CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 307 PHILIPPINES BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 308 PHILIPPINES ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 309 PHILIPPINES ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 310 PHILIPPINES ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 311 PHILIPPINES TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 312 PHILIPPINES STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 313 PHILIPPINES SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 314 PHILIPPINES MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 315 PHILIPPINES BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 316 PHILIPPINES MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 317 PHILIPPINES ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 318 PHILIPPINES PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 319 PHILIPPINES MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 320 PHILIPPINES MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 PHILIPPINES SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 322 PHILIPPINES OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 PHILIPPINES DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 324 PHILIPPINES IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 PHILIPPINES STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 326 PHILIPPINES STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 327 PHILIPPINES STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 328 MALAYSIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 MALAYSIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 330 MALAYSIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 MALAYSIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 332 MALAYSIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 333 MALAYSIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 334 MALAYSIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 335 MALAYSIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 336 MALAYSIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 337 MALAYSIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 338 MALAYSIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 339 MALAYSIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 340 MALAYSIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 341 MALAYSIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 342 MALAYSIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 343 MALAYSIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 344 MALAYSIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 345 MALAYSIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 346 MALAYSIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 347 MALAYSIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 348 MALAYSIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 349 MALAYSIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 350 MALAYSIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 351 MALAYSIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 352 MALAYSIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 353 MALAYSIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 354 MALAYSIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 355 MALAYSIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 356 MALAYSIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 357 MALAYSIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 358 MALAYSIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 359 INDONESIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 360 INDONESIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 361 INDONESIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 362 INDONESIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 363 INDONESIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 364 INDONESIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 365 INDONESIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 366 INDONESIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 367 INDONESIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 368 INDONESIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 369 INDONESIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 370 INDONESIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 371 INDONESIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 372 INDONESIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 373 INDONESIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 374 INDONESIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 375 INDONESIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 376 INDONESIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 377 INDONESIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 378 INDONESIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 379 INDONESIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 380 INDONESIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 381 INDONESIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 382 INDONESIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 383 INDONESIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 384 INDONESIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 385 INDONESIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 386 INDONESIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 387 INDONESIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 388 INDONESIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 389 INDONESIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 390 VIETNAM STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 391 VIETNAM ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 392 VIETNAM HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 393 VIETNAM STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 394 VIETNAM TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 395 VIETNAM MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 396 VIETNAM BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 397 VIETNAM ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 398 VIETNAM THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 399 VIETNAM CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 400 VIETNAM BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 401 VIETNAM ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 402 VIETNAM ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 403 VIETNAM ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 404 VIETNAM TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 405 VIETNAM STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 406 VIETNAM SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 407 VIETNAM MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 408 VIETNAM BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 409 VIETNAM MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 410 VIETNAM ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 411 VIETNAM PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 412 VIETNAM MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 413 VIETNAM MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 414 VIETNAM SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 415 VIETNAM OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 416 VIETNAM DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 417 VIETNAM IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 418 VIETNAM STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 419 VIETNAM STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 420 VIETNAM STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 421 TAIWAN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 422 TAIWAN ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 423 TAIWAN HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 424 TAIWAN STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 425 TAIWAN TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 426 TAIWAN MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 427 TAIWAN BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 428 TAIWAN ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 429 TAIWAN THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 430 TAIWAN CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 431 TAIWAN BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 432 TAIWAN ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 433 TAIWAN ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 434 TAIWAN ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 435 TAIWAN TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 436 TAIWAN STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 437 TAIWAN SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 438 TAIWAN MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 439 TAIWAN BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 440 TAIWAN MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 441 TAIWAN ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 442 TAIWAN PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 443 TAIWAN MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 444 TAIWAN MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 445 TAIWAN SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 446 TAIWAN OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 447 TAIWAN DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 448 TAIWAN IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 449 TAIWAN STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 450 TAIWAN STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 451 TAIWAN STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 452 REST OF ASIA-PACIFIC STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Liste des figures
FIGURE 1 ASIA-PACIFIC STROKE MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC STROKE MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC STROKE MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC STROKE MARKET: ASIA-PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC STROKE MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC STROKE MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA-PACIFIC STROKE MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA-PACIFIC STROKE MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 ASIA-PACIFIC STROKE MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA-PACIFIC STROKE MARKET: SEGMENTATION
FIGURE 11 RISING INCIDENCES OF STROKE DRIVE DEMAND FOR TREATMENTS IS EXPECTED TO DRIVE THE ASIA-PACIFIC STROKE MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 12 ISCHEMIC STROKE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC STROKE MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 13 ASIA-PACIFIC STROKE MARKET EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA-PACIFIC STROKE MARKET
FIGURE 16 ASIA-PACIFIC STROKE MARKET: BY TYPE, 2024
FIGURE 17 ASIA-PACIFIC STROKE MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 18 ASIA-PACIFIC STROKE MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 19 ASIA-PACIFIC STROKE MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 ASIA-PACIFIC STROKE MARKET: BY GENDER, 2024
FIGURE 21 ASIA-PACIFIC STROKE MARKET: BY GENDER, 2025 TO 2032 (USD THOUSAND)
FIGURE 22 ASIA-PACIFIC STROKE MARKET: BY GENDER, CAGR (2025- 2032)
FIGURE 23 ASIA-PACIFIC STROKE MARKET: BY GENDER, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC STROKE MARKET: BY DIAGNOSIS AND TREATMENT, 2024
FIGURE 25 ASIA-PACIFIC STROKE MARKET: BY DIAGNOSIS AND TREATMENT, 2025-2032 (USD THOUSAND)
FIGURE 26 ASIA-PACIFIC STROKE MARKET: BY DIAGNOSIS AND TREATMENT, CAGR (2025-2032)
FIGURE 27 ASIA-PACIFIC STROKE MARKET: BY DIAGNOSIS AND TREATMENT, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC STROKE MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 29 ASIA-PACIFIC STROKE MARKET: BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
FIGURE 30 ASIA-PACIFIC STROKE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 31 ASIA-PACIFIC STROKE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC STROKE MARKET: BY END USER, 2024
FIGURE 33 ASIA-PACIFIC STROKE MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 34 ASIA-PACIFIC STROKE MARKET: BY END USER, CAGR (2025-2032)
FIGURE 35 ASIA-PACIFIC STROKE MARKET: BY END USER, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC STROKE MARKET: SNAPSHOT (2024)
FIGURE 37 ASIA-PACIFIC STROKE MARKET: COMPANY SHARE 2024 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.