ヨーロッパの幹細胞療法市場、製品タイプ別(骨髄由来間葉系細胞、胎盤または臍帯幹細胞、脂肪組織由来間葉系幹細胞、その他)、タイプ別(同種幹細胞療法および自己幹細胞療法)、用途別(筋骨格障害、急性移植片対宿主病(AGVHD)、創傷および外傷、心血管疾患、手術、胃腸疾患、その他)、エンドユーザー別(病院および外科センター、治療会社、サービス会社、その他)、流通チャネル別(直接入札、サードパーティ販売業者)業界動向および2029年までの予測
市場分析と規模
がん、筋骨格系障害、神経系障害、慢性外傷、心血管系および胃腸系などの慢性疾患は、入院、長期障害、生活の質の低下、および死亡につながる可能性があります。
間葉系幹細胞は複数の臓器に浸透して統合し、心臓血管、肺、脊髄の損傷を修復し、自己免疫疾患、肝臓、骨および軟骨疾患の状態を改善します。幹細胞は、炎症、免疫不全、または組織変性によって引き起こされる感染症の治療に強力なツールです。
欧州の幹細胞治療市場の成長を牽引しているのは、慢性疾患の発生率の増加、細胞治療生産施設の GMP 認証承認の増加、バイオテクノロジー部門の成長、幹細胞ベースの治療の臨床試験の増加です。ただし、幹細胞ベースの研究コストの上昇、幹細胞治療を受ける際に直面するリスク、代替手段の利用可能性などが、市場の成長を抑制する要因になると予想されます。
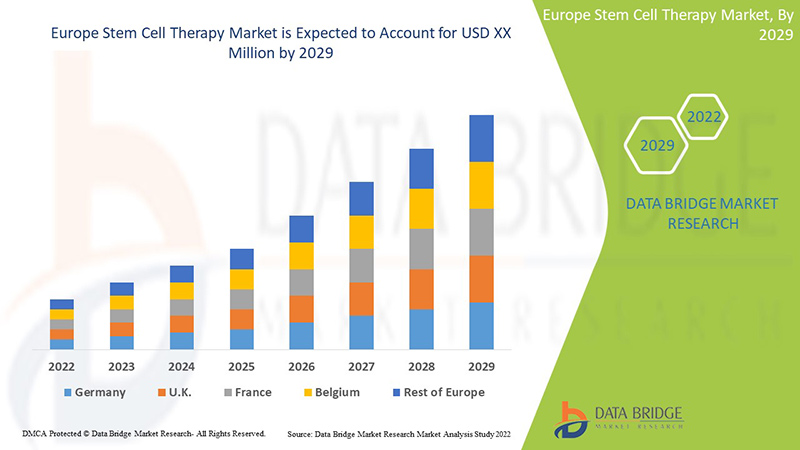
ヨーロッパの幹細胞療法は補助的であり、症状の重症度を軽減することを目的としています。データブリッジマーケットリサーチは、ヨーロッパの幹細胞療法市場は2022年から2029年の予測期間中に815万米ドルの価値に達し、年平均成長率10.0%で成長すると予測しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 (2019 - 2014 にカスタマイズ可能) |
|
定量単位 |
収益(百万米ドル) |
|
対象セグメント |
製品タイプ別(骨髄由来間葉系細胞、胎盤または臍帯幹細胞、脂肪組織由来間葉系幹細胞、その他)、タイプ別(同種幹細胞療法および自己幹細胞療法)、用途別(筋骨格障害、急性移植片対宿主病(AGVHD)、創傷および外傷、心血管疾患、手術、胃腸疾患、その他)、エンドユーザー別(病院および外科センター、治療会社、サービス会社、その他)、流通チャネル別(直接入札、サードパーティ販売業者) |
|
対象国 |
イタリア、ベルギー、チェコ共和国、ドイツ、イギリス、スペイン、ハンガリー、ポーランド |
|
対象となる市場プレーヤー |
武田薬品工業株式会社、Holostem Terapie Avanzate Srl、JCR Pharmaceuticals Co., Ltd、ANTEROGEN.CO., LTD、MEDIPOST、Orthofix Medical Inc.、BioRestorative Therapies, Inc.、STEMPEUTICS RESEARCH PVT LTD、Pluristem Inc. など |
市場の定義
幹細胞は、特殊な機能を持つ他のすべての細胞が生成される、身体の最初の材料です。身体または実験室で適切な条件下では、幹細胞は分裂して娘細胞と呼ばれる細胞を形成します。娘細胞は、血液細胞、脳細胞、心筋細胞、骨細胞など、より特殊な機能を持つ新しい幹細胞または特殊細胞(分化)になります。幹細胞に対する大きな関心は、研究者の間で関心を集めています。幹細胞を使用して病気がどのように発症し、発生するかを理解したり、細胞を置き換える健康な細胞を生成したり、新薬の安全性と有効性をテストしたりすることが、幹細胞治療が使用される科学的な理由です。
幹細胞療法は、幹細胞またはその誘導体を使用して、機能不全または損傷した組織の修復反応を促進します。これは臓器移植の次の段階であり、供給が限られているドナー臓器の代わりに細胞を使用します。脂肪組織由来間葉系幹細胞、骨髄由来間葉系幹細胞、胎盤または臍帯幹細胞などの成体幹細胞は、ほとんどの組織に少量存在します。胚性幹細胞は、3~5 日齢の胚から発生します。新たな兆候は、成体幹細胞がさまざまな種類の細胞を作成できる可能性があることを示唆しています。
欧州の幹細胞治療市場の動向
ドライバー
- 慢性疾患の有病率と発症率の上昇
慢性疾患は世界中でよく見られる健康状態です。ヨーロッパでは、成人の 3 人に 1 人が慢性疾患に苦しんでいます。慢性疾患は、多くの国民の健康と生活の質に影響を及ぼしています。がん、筋骨格障害、神経障害、慢性外傷、心血管疾患、胃腸疾患などの慢性疾患は、入院、長期障害、生活の質の低下、そして死亡につながる可能性があります。
間葉系幹細胞は複数の臓器に浸透して統合し、心臓血管、肺、脊髄の損傷を修復し、自己免疫疾患、肝臓、骨および軟骨疾患の状態を改善します。幹細胞は、炎症、免疫不全、または組織変性によって引き起こされる疾患を治療するための強力なツールです。
例えば、
- 世界保健機関(WHO)によると、2021年には世界中で約17億人が筋骨格系障害を患っていました。腰痛は筋骨格系障害の大きな負担を引き起こします。
- 幹細胞研究の研究開発の増加と資金の入手可能性
幹細胞研究は、国立衛生研究所 (NIH) の予算によって資金提供されています。民間セクターも幹細胞研究に資金を提供していますが、そのような投資は、通常、後になってから、試験および開発段階、そして初期の基礎研究の段階で行われます。幹細胞療法は非常に新しい分野であるため、公平な政府機関が監督する必要があります。FDA は慎重かつ徹底的ですが、資金獲得に絶えず苦労しており、支払いを将来の潜在的な受益者に合わせる長期的な投資を行っています。
例えば、
- 2021 年 12 月、英国では、MRC とバイオテクノロジー、および BBSRC が英国幹細胞バンクに資金を提供し、倫理的に承認され、品質管理され、保証されたヒト胚、胎児、成人の幹細胞株のリポジトリに関する研究プロジェクトを実施しました。
- 細胞治療製造施設のGMP認証承認の増加
GMP は、製品が常にその用途に適した最先端の品質基準に従って製造および管理されることを保証します。したがって、GMP の原則は、消費者や患者に一貫した品質と高い安全レベルを備えた製品を提供することに大きく貢献します。
GMP 認証を取得すれば、エラーを防止できます。認証を取得することで、製造業者は同一かつ繰り返し可能な無菌環境で細胞株を生産できます。したがって、GMP 認証はヨーロッパの幹細胞治療市場の成長を促進する可能性があります。
機会
- 医療費の増加
さらに、研究開発活動の増加と政府および民間組織による投資の増加により、市場の成長率に新たな機会がもたらされるでしょう。
例えば、
- ユーロスタットによると、2020年の欧州連合の医療費は13億960万ドルに達した。
- 市場参加者による戦略的取り組み
幹細胞療法の需要は、慢性疾患のタイムリーな治療により、ヨーロッパで増加しています。これらの有利な要因により医薬品の需要が高まり、市場の需要を満たすために、小規模および大規模な市場プレーヤーがさまざまな戦略を活用しています。
大手企業はまた、事業の円滑な運営を確保し、リスクを回避し、市場の売上の長期的な成長を高めるために、製品の発売、買収、承認、拡張、パートナーシップなどの具体的な戦略を考案しようとしています。
買収、会議、重点セグメント製品の発売など、市場プレーヤーによるこれらの戦略的取り組みは、企業の成長と製品ポートフォリオの改善に役立ち、最終的には収益の増加につながります。したがって、市場プレーヤーによるこれらの戦略的取り組みは、市場の成長を促進するのに役立つ機会を提供します。
制約/課題
- 幹細胞治療研究のコスト上昇
Stem cell therapy is a developing and novel treatment option for treating several disorders. Sometimes, the cost of the therapy is a concern for several conditions. The stem-cell therapy treatment procedures. The stem cell field is still highly specialized and has not been adopted by the mainstream and insurance companies. The cost of stem cell therapy-based research therapy is not covered by medical insurance. These costs are pushed on patients. Therefore, the present high cost is expected to show a descending trend.
For instance,
- In 2021, the data by Gift of Life, a U.K. sister charity to Podari Zhizn, stated that the cost of stem cell therapy for musculoskeletal disorders is USD 4861.44 to USD 5401.60
- The risks faced while undergoing stem cell therapy
Specific risks are observed by stem cell therapists and research scientists while developing the stem cell therapeutic models. The main challenge faced while using stem cell therapy is optimal cell sourcing. Stem cell therapy is well established and rapidly growing in developed countries; however, certain risks shield the growth of this therapy in developing countries. The criteria limit the use of stem cell therapy. Hence, there are certain risks observed for patients undergoing stem cell therapy.
For instance,
- The risks faced while undergoing stem cell therapy are genetic instability, tumor formation, inappropriate stem cell formation, immune rejection of transplanted stem cells, hemorrhage during neurosurgery, and postoperative infection
- The risks faced after stem cell transplantation is improper distribution and localization of stem cell after transplant. Pluripotent stem cells can form teratoma (tumor)
- Stringent regulations
The regulatory guidelines for stem cell therapeutics are strict compared to older ones. The manufacturers have to make specific product changes before approval, which is expected to cause delays. The companies have to carefully review the product specifications and certifications before categorizing their product according to the Food and Drug Administration (FDA) and the European Union (E.U.). Every country has its regulatory authority responsible for enforcing rules and regulations for drug development, licensing, registration, manufacturing, marketing, and labeling of pharmaceutical products.
FDA's Center for Biologics Evaluation & Research (CBER) regulates human tissues, cells, and tissue-based products intended for transplantation, including hematopoietic stem cells.
For instance,
- In the U.K., the Medicines and Healthcare Products Regulatory Agency (MHRA) regulates and provides guidelines for stem cell therapy; when stem cell therapy is considered to be a Medicinal Product (M.P.), for quality and safety (Q and S), testing regulations
The Europe stem cell therapy market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on t Europe stem cell therapy market, contact Data Bridge Market Research for an Analyst Brief; our team will help you make an informed market decision to achieve market growth.
Patient Epidemiology Analysis
The Europe stem cell therapy market also provides you with detailed market analysis for patient analysis, prognosis, and cures. Prevalence, incidence, mortality, and adherence rates are some of the data variables available in the report. Direct or indirect impact analyses of epidemiology to the market growth are analyzed to create a more robust and cohort multivariate statistical model for forecasting the market during the growth period.
Impact of COVID-19 on the Europe Stem Cell Therapy Market
During the pandemic, stem cell therapy had a remarkable effect on reducing mortality and morbidity of patients with COVID-19. Further large-scale studies are needed to approve these results. A protocol for stem cell therapy in COVID-19 infection should be defined to achieve the best possible clinical outcomes. Clinical trials were conducted during COVID-19.
Recent Development
- In September 2020, Takeda Pharmaceutical Company Limited. had received product approval from the Japan Ministry of Health, Labour and Welfare to manufacture and market Alofisel (darvadstrocel) and adipose-derived mesenchymal stem cells for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn's disease (CD). The approval received would result in post-market approval and product launch. This is expected to increase the market growth.
Europe Stem Cell Therapy Market Scope
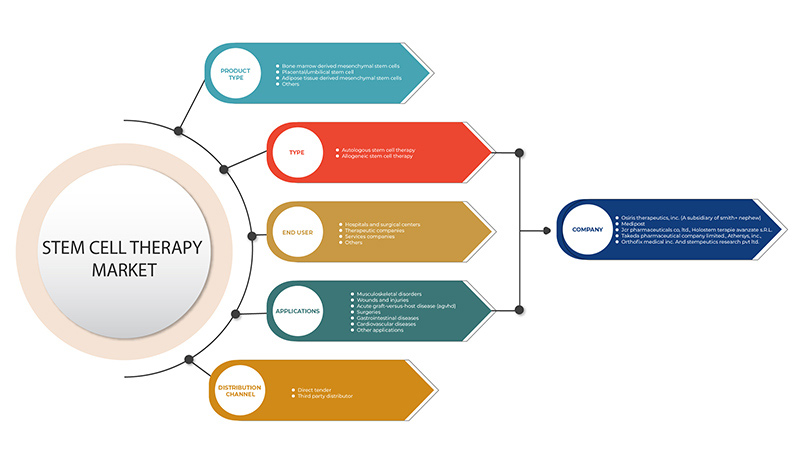
The Europe stem cell therapy market is segmented into five segments based on product type, type, application, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Bone Marrow Derived Mesenchymal Stem Cells
- Placental or Umbilical Stem Cell
- Adipose Tissue Derived Mesenchymal Stem Cells
- Others
On the basis of product type, the Europe stem cell therapy market is segmented into bone marrow derived mesenchymal stem cells, placental or umbilical stem cell, adipose tissue derived mesenchymal stem cells, and others.
Type
- Allogeneic Stem Cell Therapy
- Autologous Stem Cell Therapy
On the basis of type, the Europe stem cell therapy market is segmented into allogenic stem cell therapy, and autologous stem cell therapy.
Application
- Musculoskeletal Disorders
- Wounds and Injuries
- Acute Graft-Versus-Host Disease (AGVHD)
- Surgeries
- Gastrointestinal Diseases
- Cardiovascular Diseases
- Others
On the basis of application, the Europe stem cell therapy market is segmented into musculoskeletal disorders, wounds and injuries, acute graft-versus-host disease (AGVHD), surgeries, gastrointestinal diseases, cardiovascular diseases, and others.
End User
- Hospitals and Surgical Centers
- Therapeutic Companies
- Services Companies
- Others
On the basis of end user, the Europe stem cell therapy market is segmented into hospitals and surgical centers, therapeutic companies, services companies, and others.
Distribution Channel
- Direct Tenders
- Third Party Distributors
On the basis of distribution channel, the Europe stem cell therapy market is segmented into direct tender, and third party distributors.
Europe Stem Cell Therapy Market Regional Analysis/Insights
The Europe stem cell therapy market is analyzed, and market size insights and trends are provided by regions, product type, type, application, end user, and distribution channel, as referenced above.
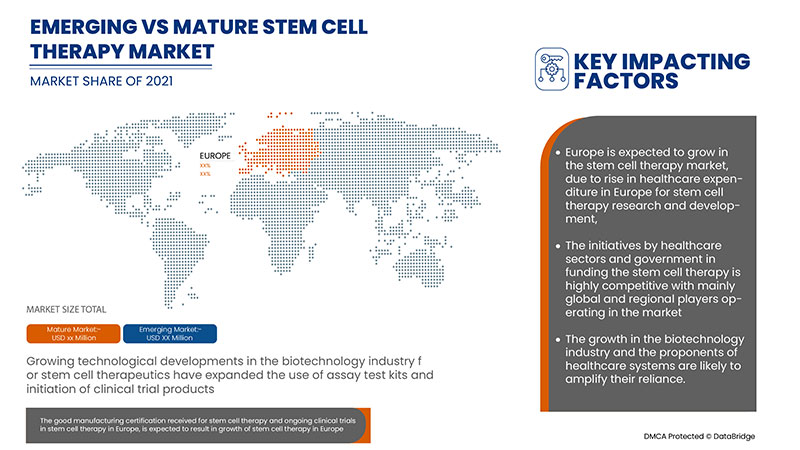
Some of the countries covered in the Europe stem cell therapy market report are Italy, Belgium, the Czech Republic, Germany, the U.K., Spain, Hungary, and Poland. Italy is expected to dominate the market due to a rise in approval for clinical trials in stem cell research and developments in the field of human stem cells.
The country section of the report also provides individual market impacting factors and changes in regulations in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of European brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Stem Cell Therapy Market Share Analysis
ヨーロッパの幹細胞療法市場の競争状況は、競合他社の詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、ヨーロッパでのプレゼンス、生産拠点と施設、生産能力、会社の強みと弱み、製品の発売、製品の幅と広さ、アプリケーションの優位性などがあります。提供されている上記のデータ ポイントは、ヨーロッパの幹細胞療法市場に関連する会社の焦点にのみ関連しています。
ヨーロッパの幹細胞治療市場で活動している主要企業としては、武田薬品工業株式会社、Holostem Terapie Avanzate Srl、JCR Pharmaceuticals Co., Ltd、ANTEROGEN.CO., LTD、MEDIPOST、Orthofix Medical Inc.、BioRestorative Therapies, Inc.、STEMPEUTICS RESEARCH PVT LTD、Pluristem Inc.などが挙げられます。
研究方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。市場データは、市場統計モデルとコヒーレント モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。 DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数の市場への影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。これとは別に、データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、企業市場シェア分析、測定基準、ヨーロッパと地域、ベンダー シェア分析が含まれます。調査方法の詳細については、お問い合わせをドロップして、当社の業界の専門家にご相談ください。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE STEM CELL THERAPY MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 EUROPE STEM CELL THERAPY MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR THE EUROPE STEM CELL THERAPY MARKET
7 EUROPE STEM CELL THERAPY MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 THE RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
8.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT AND AVAILABILITY OF FUNDING FOR STEM CELL RESEARCH
8.1.3 GROWING BIOTECHNOLOGY SECTOR
8.1.4 RISE IN GMP CERTIFICATION APPROVALS FOR CELL THERAPY PRODUCTION FACILITIES
8.1.5 RISE IN CLINICAL TRIALS FOR STEM-CELL-BASED THERAPIES
8.2 RESTRAINTS
8.2.1 THE RISE IN COST OF STEM-CELL-BASED THERAPY RESEARCH
8.2.2 THE RISKS FACED WHILE UNDERGOING STEM CELL THERAPY
8.2.3 ETHICAL CONCERNS RELATED TO STEM CELL THERAPY RESEARCH
8.2.4 AVAILABILITY OF ALTERNATIVES
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
8.3.2 RISE IN HEALTHCARE EXPENDITURE
8.3.3 THE EMERGENCE OF INDUCED PLURIPOTENT STEM CELLS (IPSCS)
8.4 CHALLENGES
8.4.1 THE LACK OF SKILLED PROFESSIONALS REQUIRED FOR STEM CELL THERAPY
8.4.2 STRINGENT REGULATIONS
9 EUROPE STEM CELL THERAPY MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 BONE MARROW DERIVED MESENCHYMAL STEM CELLS
9.3 PLACENTAL/UMBILICAL STEM CELL
9.4 ADIPOSE TISSUE DERIVED MESENCHYMAL STEM CELLS
9.5 OTHERS
10 EUROPE STEM CELL THERAPY MARKET, BY TYPE
10.1 OVERVIEW
10.2 ALLOGENEIC STEM CELL THERAPY
10.2.1 MUSCULOSKELETAL DISORDERS
10.2.2 WOUNDS AND INJURIES
10.2.3 ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD)
10.2.4 SURGERIES
10.2.5 GASTROINTESTINAL DISEASES
10.2.6 OTHER APPLICATION
10.3 AUTOLOGOUS STEM CELL THERAPY
10.3.1 CARDIOVASCULAR DISEASES
10.3.2 GASTROINTESTINAL DISEASES
10.3.3 OTHER APPLICATION
11 EUROPE STEM CELL THERAPY MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 MUSCULOSKELETAL DISORDERS
11.3 WOUNDS AND INJURIES
11.4 ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD)
11.5 SURGERIES
11.6 GASTROINTESTINAL DISEASES
11.7 CARDIOVASCULAR DISEASES
11.8 OTHER APPLICATION
12 EUROPE STEM CELL THERAPY MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL AND SURGICAL CENTERS
12.3 THERAPEUTIC COMPANIES
12.4 SERVICES COMPANIES
12.5 OTHERS
13 EUROPE STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTORS
14 EUROPE STEM CELL THERAPY MARKET, BY REGION
14.1 EUROPE
14.1.1 ITALY
14.1.2 BELGIUM
14.1.3 CZECH REPUBLIC
14.1.4 GERMANY
14.1.5 U.K.
14.1.6 SPAIN
14.1.7 HUNGARY
14.1.8 POLAND
15 EUROPE STEM CELL THERAPY MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 OSIRIS THERAPEUTICS, INC. (A SUBSIDIARY OF SMITH+NEPHEW) (2021)
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 JCR PHARMACEUTICALS CO., LTD ( (2021)
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 ORTHOFIX MEDICAL INC. (2021)
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.4 MEDIPOST (2021)
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.5 TAKEDA PHARMACEUTICAL COMPANY LIMITED (2021)
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CORESTEM, INC. (2021)
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 PHARMICELL CO., LTD. (2021)
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.8 ANTEROGEN.CO., LTD (2021)
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENTS
17.9 ATHERSYS, INC.(2021)
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
17.1 BRAINSTORM CELL LIMITED (2021)
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENTS
17.11 BIORESTORATIVE THERAPIES, INC. (2021)
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 HOLOSTEM TERAPIE AVANZATE S.R.L. (2021)
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENTS
17.13 INTERNATIONAL STEMCELL CORPORATION (2021)
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 MESOBLAST LTD (2021)
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENTS
17.15 PLURISTEM INC.(2021)
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 STEMPEUTICS RESEARCH PVT LTD
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENTS
17.17 U.S. STEM CELL, INC. (2021)
17.17.1 COMPANY SNAPSHOT
17.17.2 REVENUE ANALYSIS
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
表のリスト
TABLE 1 EUROPE STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE BONE MARROW DERIVED MESENCHYMAL STEM CELLS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 EUROPE PLACENTAL/UMBILICAL STEM CELL IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE ADIPOSE TISSUE DERIVED MESENCHYMAL STEM CELLS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE OTHERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 EUROPE ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 EUROPE ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE MUSCULOSKELETAL DISORDERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE WOUNDS AND INJURIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD) IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE SURGERIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE GASTROINTESTINAL DISEASES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE CARDIOVASCULAR DISEASES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE OTHER APPLICATION IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 20 EUROPE HOSPITAL AND SURGICAL CENTERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE THERAPEUTIC COMPANIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE SERVICES COMPANIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE OTHERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 25 EUROPE DIRECT TENDER IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE THIRD PARTY DISTRIBUTORS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE STEM CELL THERAPY MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 28 EUROPE STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 29 EUROPE STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 30 EUROPE ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 33 EUROPE STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 34 EUROPE STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 35 ITALY STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 36 ITALY STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 37 ITALY ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 38 ITALY AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 39 ITALY STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 40 ITALY STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 ITALY STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 42 BELGIUM STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 43 BELGIUM STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 BELGIUM ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 45 BELGIUM AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 46 BELGIUM STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 47 BELGIUM STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 BELGIUM STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 49 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 50 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 51 CZECH REPUBLIC ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 52 CZECH REPUBLIC AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 53 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 54 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 GERMANY STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 57 GERMANY STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 58 GERMANY ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 59 GERMANY AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 60 GERMANY STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 61 GERMANY STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 62 GERMANY STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 63 U.K. STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 64 U.K. STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 65 U.K. ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 66 U.K. AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 U.K. STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 68 U.K. STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 69 U.K. STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 70 SPAIN STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 SPAIN STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 72 SPAIN ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 73 SPAIN AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 SPAIN STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 75 SPAIN STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 76 SPAIN STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 77 HUNGARY STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 HUNGARY STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 HUNGARY ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 80 HUNGARY AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 81 HUNGARY STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 82 HUNGARY STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 83 HUNGARY STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 84 POLAND STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 85 POLAND STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 86 POLAND ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 87 POLAND AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 88 POLAND STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 89 POLAND STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 90 POLAND STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
図表一覧
FIGURE 1 EUROPE STEM CELL THERAPY MARKET : SEGMENTATION
FIGURE 2 EUROPE STEM CELL THERAPY MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE STEM CELL THERAPY MARKET: DROC ANALYSIS
FIGURE 4 EUROPE STEM CELL THERAPY MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE STEM CELL THERAPY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE STEM CELL THERAPY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE STEM CELL THERAPY MARKET: DBMR POSITION GRID
FIGURE 8 EUROPE STEM CELL THERAPY MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE STEM CELL THERAPY MARKET: END USER COVERAGE GRID
FIGURE 10 EUROPE STEM CELL THERAPY MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE EUROPE STEM CELL THERAPY MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN CLINICAL TRIALS, GMP CERTIFICATION AND PRODUCT APPPROVALS IS EXPECTED TO DRIVE THE EUROPE STEM CELL THERAPY MARKET FROM 2022 TO 2029
FIGURE 13 PRODUCT TYPE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE EUROPE STEM CELL THERAPY MARKET FROM 2022 & 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE STEM CELL THERAPY MARKET
FIGURE 15 NUMBER AND AGES OF PEOPLE 65 OR OLDER WITH ALZHEIMER'S DEMENTIA IN 2022
FIGURE 16 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 17 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, 2021
FIGURE 18 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 19 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 20 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 21 EUROPE STEM CELL THERAPY MARKET: BY TYPE, 2021
FIGURE 22 EUROPE STEM CELL THERAPY MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 23 EUROPE STEM CELL THERAPY MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 24 EUROPE STEM CELL THERAPY MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, 2021
FIGURE 26 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 27 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 28 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 29 EUROPE STEM CELL THERAPY MARKET: BY END USER, 2021
FIGURE 30 EUROPE STEM CELL THERAPY MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 31 EUROPE STEM CELL THERAPY MARKET: BY END USER, CAGR (2022-2029)
FIGURE 32 EUROPE STEM CELL THERAPY MARKET: BY END USER, LIFELINE CURVE
FIGURE 33 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 34 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 35 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 36 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 37 EUROPE STEM CELL THERAPY MARKET: SNAPSHOT (2021)
FIGURE 38 EUROPE STEM CELL THERAPY MARKET: BY COUNTRY (2021)
FIGURE 39 EUROPE STEM CELL THERAPY MARKET: BY COUNTRY (2022 & 2029)
FIGURE 40 EUROPE STEM CELL THERAPY MARKET: BY COUNTRY (2021 & 2029)
FIGURE 41 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE (2022 & 2029)
FIGURE 42 EUROPE STEM CELL THERAPY MARKET: COMPANY SHARE 2021 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。













