North America Lysosomal Storage Disorder Drugs Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
4.23 Billion
USD
8.82 Billion
2024
2032
USD
4.23 Billion
USD
8.82 Billion
2024
2032
| 2025 –2032 | |
| USD 4.23 Billion | |
| USD 8.82 Billion | |
|
|
|
North America Lysosomal Storage Disorder Drugs Market Segmentation, By Type of Disorder Gaucher DiseaseFabry DiseasePompe Disease, Mucopolysaccharidosis (MPS), Niemann-Pick DiseaseKrabbe Disease, and Others), Type (Enzyme Replacement Therapy (ERT), Substrate Reduction Therapy (SRT), Chaperone Therapy, and Others), Drugs (Imiglucerase, Agalsidase Beta, Idursulfase, Alglucosidase Alpha, Velaglucerase, Taliglucerase Alfa, Laronidase, Agalsidase Alpha, Galsulfase, Avalglucosidase Alfa, and Others), Route of Administration (Intravenous (IV), Subcutaneous (SC), Oral, and Others), Age Group (Pediatric, Adults, and Geriatric), Gender (Male and Female), Distribution Channel (Hospital Pharmacies, Drugs Stores and Retail Pharmacies, and Online Pharmacies) - Industry Trends and Forecast to 2032
Lysosomal Storage Disorder Drugs Market Analysis
The North America lysosomal storage disorder drugs market encompasses the commercial sector for pharmaceuticals developed to diagnose, treat, and manage various lysosomal storage disorders, which are rare genetic conditions typically characterized by the accumulation of undigested substances within lysosomes due to enzyme deficiencies. This market includes a range of therapeutic products, such as enzyme replacement therapies, substrate reduction therapies, and gene therapies, aimed at addressing the diverse clinical manifestations of LSDs, such as Gaucher disease, Fabry disease, and Pompe disease. With increasing awareness, advancements in research and technology, and a growing number of pipeline therapies, this market is poised for significant expansion. In addition, rising healthcare expenditure and initiatives to improve access to rare disease treatments further drive market growth, presenting both opportunities and challenges for pharmaceutical companies and healthcare providers.
Lysosomal Storage Disorder Drugs Market Size
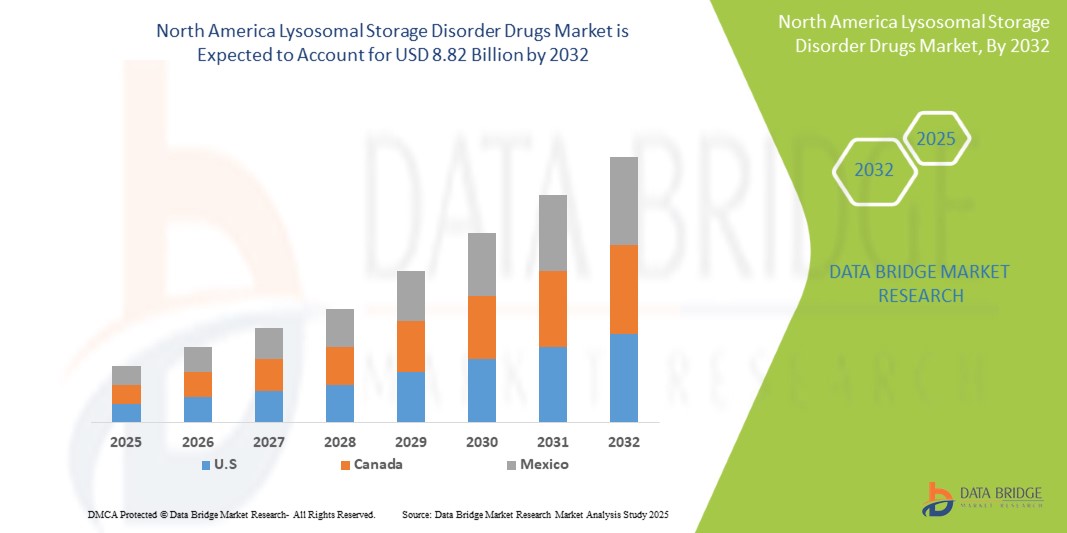
North America lysosomal storage disorder drugs market size was valued at USD 4.23 billion in 2024 and is projected to reach USD 8.82 billion by 2032, growing with a CAGR of 9.7% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework.
North America Lysosomal Storage Disorder Drugs Market Trends
“Biotechnology Advancements for Lysosomal Storage Disorders Treatments”
Advancements in biotechnology have played a pivotal role in transforming the treatment landscape for Lysosomal Storage Disorders (LSDs), providing patients with more effective and tailored treatment options. Enzyme Replacement Therapy (ERT) has revolutionized the treatment of Lysosomal Storage Disorders (LSDs) by directly addressing the enzyme deficiencies that cause these conditions. In LSD patients, the lack of specific enzymes leads to the accumulation of toxic substances in cells, damaging organs and tissues. ERT works by administering synthetic enzymes to compensate for the missing ones, improving metabolic functions and alleviating symptoms. Over the years, ERT has advanced significantly, with new, more effective formulations and delivery methods that improve enzyme absorption and reduce side effects. These innovations have resulted in better clinical outcomes, this trend slowed disease progression, reduced organ damage, and enhanced patient quality of life.
Report Scope and Lysosomal Storage Disorder Drugs Market Segmentation
|
Attributes |
Lysosomal Storage Disorder Drugs Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, and Mexico |
|
Key Market Players |
Sanofi (France), BioMarin (U.S.), Pfizer Inc. (U.S.), Amicus Therapeutics, Inc. (U.S.), Takeda Pharmaceutical Company Limited (Japan), Ultragenyx Pharmaceutical Inc. (U.S.), Orchard Therapeutics plc (U.K.), Spur Therapeutics (U.K.), Sangamo Therapeutics (U.S.), Protalix BioTherapeutics Inc. (Israel), Forge Biologics (U.S.), Denali Therapeutics (U.S.), REGENXBIO INC. (U.S.), and JCR Pharmaceuticals Co., Ltd. (Japan) among others |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Lysosomal Storage Disorder Drugs Market Definition
Lysosomal storage disorder drugs refer to a category of pharmaceuticals specifically developed to treat Lysosomal Storage Disorders (LSDs), which are a group of rare genetic conditions caused by deficiencies in specific enzymes responsible for breaking down complex molecules within lysosomes. These drugs aim to address the underlying metabolic abnormalities associated with LSDs by either replacing the missing enzymes (enzyme replacement therapy), by enhancing the body's ability to produce the enzymes, or by modifying the substrates that accumulate due to enzyme deficiency (substrate reduction therapy). By improving enzymatic function or reducing the toxic build-up of substrates, these treatments can alleviate symptoms, slow disease progression, and ultimately improve the quality of life for individuals affected by these challenging conditions.
Lysosomal Storage Disorder Drugs Market Dynamics
Drivers
- Emerging Personalized Medicine for Lysosomal Storage Disorders
Emerging personalized medicine is transforming the landscape of treatment for Lysosomal Storage Disorders (LSDs) by offering therapies specifically tailored to the individual genetic profiles of patients. LSDs are caused by genetic mutations that affect enzyme function, and these mutations can vary widely between patients. Personalized treatments involve analyzing a patient’s unique genetic makeup to develop a more targeted approach, optimizing the efficacy of therapies such as enzyme replacement or gene therapy. This precision allows for better matching of treatments with the patient's specific needs, minimizing side effects and enhancing therapeutic outcomes. As advancements in genetic testing and technology progress, healthcare providers are able to identify the most suitable interventions, potentially improving response rates and overall treatment success. The shift toward personalized medicine is accelerating the development of customized LSD treatments, which promise to revolutionize disease management. With these innovations, the global lysosomal storage disorders drug market is witnessing increased demand for more effective, individualized treatment options. Personalized medicine is thus a key driver of market growth, as it boosts treatment effectiveness and enhances patient outcomes, fostering greater confidence in therapeutic options.
For instance,
In February 2023, according to the article published by NCBI, Emerging personalized medicine uses an individual’s genetic profile to guide decisions related to the prevention, diagnosis, and treatment of diseases. This approach enables more precise and effective therapies for Lysosomal Storage Disorders (LSDs), tailoring treatments to specific genetic mutations. As personalized medicine becomes more prevalent, it drives the demand for targeted LSD therapies, fueling growth in the global lysosomal storage disorders drug market.
- Increased Government Funding for Research into Rare Disease Treatments
Government funding and grants for research into rare disease treatments are crucial for advancing the development of therapies for conditions such as Lysosomal Storage Disorders (LSDs). Rare diseases, often lacking commercial viability due to small patient populations, pose significant challenges in drug development. To address this, governments worldwide are increasingly allocating funds to incentivize research in these underserved areas. Financial support in the form of grants, subsidies, and tax credits helps pharmaceutical companies and research institutions overcome the high costs and risks associated with developing treatments for rare diseases. Furthermore, governments often fast-track regulatory processes for these treatments, recognizing the urgent need for solutions. By reducing the financial burden on researchers and companies, these funding mechanisms enable the exploration of innovative therapies, such as enzyme replacement and gene therapies that would otherwise face significant barriers to development.
For instance,
In August 2023, according to the article published by National Organization for Rare Diseases, The National Organization for Rare Disorders announced over USD 100,000 in grant funding for rare disease research, highlighting increased government support for advancing treatments. These funds enable researchers to explore innovative therapies for rare diseases like Lysosomal Storage Disorders (LSDs). Such financial backing accelerates drug development, driving growth in the global LSD drug market by fostering new treatment options.
Opportunities
- Increasing Number of Pipeline Drugs
The increasing number of drugs in the pipeline for Lysosomal Storage Disorders (LSDs) represents a significant opportunity for the global lysosomal storage disorders drugs market, potentially leading to improved treatment options and enhanced patient outcomes. As researchers and pharmaceutical companies enhance their understanding of these disorders, they are developing a diverse array of novel therapies, including gene therapies, substrate reduction therapies, and small molecule drugs. This expanded pipeline reflects a growing recognition of the unmet medical needs within the LSD community and promises to offer patients a wider variety of treatment modalities tailored to their specific conditions. The introduction of innovative therapies can enhance patient adherence to treatment, reduce disease burden, and ultimately improve the quality of life for individuals living with these disorders.
For instance,
In June 2024, Ultragenyx announced the Planning to file for accelerated approval of UX111 for the treatment of Sanfilippo Syndrome Type A (MPS IIIA). UX111 is a novel in vivo gene therapy in Phase 1/2/3 development for Sanfilippo syndrome type A (MPS IIIA), a rare fatal lysosomal storage disease with no approved treatment that primarily affects the central nervous system.
- Growing Emphasis on Early Diagnosis
The growing emphasis on early diagnosis of Lysosomal Storage Disorders (LSDs) presents a significant opportunity for the global lysosomal storage disorders drugs market by facilitating timely interventions that can greatly enhance patient outcomes. As healthcare systems place greater emphasis on early detection through improved screening programs, genetic testing, and advancements in diagnostic technologies, more patients are likely to be diagnosed at an earlier stage of their diseases. This early intervention allows for better management of symptoms but increases the potential efficacy of existing and emerging therapies. The result of this shift is a broader patient base that requires and can benefit from treatment options, ultimately driving demand within the lysosomal storage disorders drug market.
For instance,
In February 2023, according to an article, ‘Lysosomal storage disorders: from biology to the clinic with reference to India’, early diagnosis is the most critical part of the management of LSDs as it provides the opportunity for therapeutic intervention, precise genetic counselling, prenatal diagnosis and a better outcome for the patient.
Restraints/Challenges
- Lack of Awareness Among Healthcare Professionals and Patients
The lack of awareness among healthcare professionals and patients about Lysosomal Storage Disorders (LSDs) presents a significant barrier to early diagnosis, treatment, and effective management. Many healthcare providers, especially in regions with limited exposure to rare diseases, often fail to recognize the symptoms of LSDs, which are diverse and overlap with other more common conditions. This lack of knowledge leads to delayed diagnoses, misdiagnosis, and ineffective treatment, exacerbating the severity of the disease. For patients, especially those in resource-poor or underserved areas, the lack of awareness prevents early intervention, leaving them unaware of potential therapies. The complexity and rarity of LSDs further complicate the situation, as patients and healthcare professionals may not fully understand the importance of early treatment or the availability of specialized care options. Fewer patients are diagnosed and treated in time, leading to suboptimal clinical outcomes. This lack of awareness limits the demand for LSD-specific treatments and hinders the overall growth of the global lysosomal storage disorders drugs market.
For instance,
In January 2024, according to the article published by Wiley, there’s a significant diagnostic delay of around 15 years from symptom onset to diagnosis is common in adult LSD cases due to overlapping clinical phenotypes and varying severity. This delay highlights the lack of awareness among healthcare professionals, particularly those treating adult patients. Such restraints hinder early detection and timely treatment, restricting the growth of the global lysosomal storage disorders drugs market.
- Prolonged Drug Approval Procedures
긴 약물 승인 절차는 북미 리소좀 저장 장애 약물 시장에 상당한 제약을 가하고, 절실히 필요한 치료법의 도입을 늦춥니다. 특히 LSD와 같은 희귀 질환의 경우 신약에 대한 규제 승인을 얻는 절차에는 수많은 임상 시험, 광범위한 문서화, FDA 및 EMA와 같은 규제 기관의 엄격한 평가가 포함됩니다. 이러한 승인 일정은 종종 안전성, 효능 및 잠재적인 장기적 효과에 대한 철저한 평가가 필요하기 때문에 연장됩니다. LSD의 경우 이러한 질병의 희귀성과 복잡성은 임상 시험에 대한 환자 집단이 제한되어 견고한 데이터를 생성하기 어렵기 때문에 또 다른 과제를 추가합니다. 이러한 희귀 질환에 대한 전문적 치료법의 필요성과 확립된 표준의 부족은 승인 절차를 복잡하게 만듭니다. 결과적으로 약물 개발자는 장기간의 대기 기간, 새로운 치료법 출시 지연 및 추가 비용에 직면하게 되며, 이는 제약 회사에 낙담할 수 있습니다. 결과적으로, 긴 승인 절차로 인해 환자에게 혁신적인 치료법을 제공하는 것이 늦어지고, 시장 성장이 감소하며, LSD에 대한 효과적인 치료법의 접근성이 방해를 받습니다. 이러한 규제 지연은 궁극적으로 글로벌 리소좀 저장 장애 약물 시장에 큰 제약으로 작용합니다.
예를 들어,
Drugs.com에서 발행한 기사에 따르면 2024년 8월, 약물의 연구, 개발 및 승인 절차는 일반적으로 12~15년이 걸립니다. 이 연장된 타임라인은 새로운 치료법의 도입을 지연시키고 제약 회사의 비용을 증가시킵니다. 결과적으로, 긴 약물 승인 절차는 치료 가용성 속도를 제한하여 글로벌 LSD 약물 시장의 성장을 억제합니다.
이 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 창출처, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
리소좀 저장 장애 약물 시장 범위
시장은 장애 유형, 유형, 약물, 투여 경로, 연령대, 성별 및 유통 채널을 기준으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내리는 데 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
장애의 종류
- 고셰병
- 1형
- 3형
- 2형
- 파브리병
- 폼페병
- 유아발병형 폼페병
- 후기 발병 폼페
- 점액다당증(MPS)
- MPS 1
- MPS II
- MPS 4
- MPS 6세
- MPS III
- 니만-픽병
- C형
- B형
- A형
- 크라베병
- 기타
유형
- 효소 대체 요법 (ERT)
- 기질 감소 요법(SRT)
- 보호자 치료
- 기타
약제
- 이미글루세라제
- 아갈시다제 베타
- 이두르설파제
- 알글루코시다제 알파
- 벨라글루세라제
- 탈리글루세라제 알파
- 라로니다제
- 아갈시다제 알파
- 갈설파제
- 아발글루코시다제 알파
- 기타
투여 경로
- 정맥 주사 (IV)
- 피하(SC)
- 경구
- 기타
연령대
- 소아과
- 성인
- 노인
성별
- 남성
- 여성
유통 채널
- 병원 약국
- 약국 및 소매 약국
- 온라인 약국
리소좀 저장 장애 약물 시장 지역 분석
위에 언급된 대로, 질병 유형, 약물, 투여 경로, 연령대, 성별, 유통 채널별로 시장을 분석하고 시장 규모에 대한 통찰력과 추세를 제공합니다.
이 시장에서 다루는 국가는 미국, 캐나다, 멕시코입니다.
미국은 첨단 의료 인프라, 높은 약물 채택률, 상당한 연구 개발 투자, 대규모 환자 인구 덕분에 북미 리소좀 저장 장애 약물 시장에서 주도적인 역할을 하며 가장 빠르게 성장하는 국가가 될 것으로 예상됩니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변화를 제공합니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 트렌드 및 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 북미 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
리소좀 저장 장애 약물 시장 점유율
시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 북미 지역 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 애플리케이션 우세입니다. 위에 제공된 데이터 포인트는 시장과 관련된 회사의 초점에만 관련이 있습니다.
시장에서 운영되는 리소좀 저장 장애 약물 시장 리더는 다음과 같습니다.
- 사노피(프랑스)
- 바이오마린(미국)
- 파이저 주식회사(미국)
- Amicus Therapeutics, Inc. (미국)
- 다케다제약 주식회사(일본)
- Ultragenyx Pharmaceutical Inc. (미국)
- Orchard Therapeutics plc (영국)
- 스퍼 테라퓨틱스(영국)
- 샌가모 테라퓨틱스(미국)
- Protalix BioTherapeutics Inc. (이스라엘)
- Forge Biologics (미국)
- 데날리 테라퓨틱스(미국)
- REGENXBIO INC. (미국)
- JCR Pharmaceuticals Co., Ltd. (일본)
리소좀 저장 장애 약물 시장의 최신 개발
- 2024년 10월, Amicus Therapeutics는 파브리병을 치료하는 자사 약물 Galafold(migalastat)에 대한 합의를 발표했습니다. 이 합의는 진행 중인 특허 분쟁을 해결하고 소송 간섭 없이 약물 마케팅을 계속한다는 것을 확인합니다. 여러 당사자와의 이 합의는 지적 재산권을 보호하는 동시에 Galafold의 가용성과 개발에 안정성을 제공하는 것을 목표로 합니다.
- 2024년 6월, Amicus Therapeutics는 파브리병 관리를 위한 혁신적인 치료법인 Pombiliti(miglustat)에 대해 Prix Galien UK Award를 수상했습니다. 이 상은 제약 혁신의 우수성을 인정하고 희귀 유전적 질환이 있는 환자의 삶을 개선하는 데 있어 Pombiliti의 영향을 강조합니다. 이 찬사는 희귀 질환 치료 분야에서 Amicus의 리더십을 강조합니다.
- 2024년 4월, Forge Biologics는 2024년 5월 ASGCT 27th Annual Meeting에서 9회 발표할 것이라고 발표했으며, 여기에는 늦은 구두 발표와 3개의 기술 세션이 포함됩니다. 발표에서는 공정 개발, 분자적 발전, 임상적 업데이트에 대해 다룰 예정이며, 여기에는 크라베병에서 FBX-101에 대한 중요한 임상 결과가 포함됩니다.
- 2023년 11월, Chiesi Group은 이탈리아, 호주, 미국 등 여러 지역에서 Great Place to Work-Certified 조직으로 재인증을 받았습니다. 직원의 85% 응답률로 Chiesi는 83%의 전반적인 만족도를 달성했으며, 이는 직원의 웰빙과 성장에 초점을 맞춘 긍정적이고 포용적이며 협력적인 업무 환경을 조성하려는 노력을 반영합니다.
- 2024년 1월, 신경퇴행성 질환 및 리소좀 저장 질환 치료를 위해 혈액-뇌 장벽을 통과하는 치료법을 개발하는 바이오제약 회사인 Denali Therapeutics Inc.는 2024년의 진행 상황과 이정표를 발표했습니다. CEO인 Ryan Watts 박사는 1월 9일 제42회 JP Morgan Healthcare Conference에서 기업 프레젠테이션을 통해 이러한 개발 사항을 강조했습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PORTER’S FIVE FORCES
4.2 PIPELINE ANALYSIS
5 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN NORTH AMERICA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDERS TREATMENTS
6.1.2 EMERGING PERSONALIZED MEDICINE FOR LYSOSOMAL STORAGE DISORDERS
6.1.3 INCREASED GOVERNMENT FUNDING FOR RESEARCH INTO RARE DISEASE TREATMENTS
6.1.4 COLLABORATIONS AND PARTNERSHIPS BETWEEN PHARMACEUTICAL COMPANIES AND RESEARCH INSTITUTIONS
6.2 RESTRAINTS
6.2.1 LACK OF AWARENESS AMONG HEALTHCARE PROFESSIONALS AND PATIENTS
6.2.2 PROLONGED DRUG APPROVAL PROCEDURES
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE DRUGS
6.3.2 GROWING EMPHASIS ON EARLY DIAGNOSIS
6.3.3 ADVANCEMENTS IN GENE THERAPY FOR THE TREATMENT OF LYSOSOMAL STORAGE DISORDERS
6.4 CHALLENGES
6.4.1 SIGNIFICANT EXPENSES RELATED TO THE TREATMENT OF THE DISEASE
6.4.2 NARROW PATIENT BASE SUFFERING FROM LYSOSOMAL STORAGE DISORDERS
7 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
7.1 OVERVIEW
7.2 GAUCHER DISEASE
7.2.1 TYPE 1
7.2.2 TYPE 3
7.2.3 TYPE 2
7.3 FABRY DISEASE
7.4 POMPE DISEASE
7.4.1 INFANTILE-ONSET POMPE
7.4.2 LATE-ONSET POMPE
7.5 MUCOPOLYSACCHARIDOSIS (MPS)
7.5.1 MPS I
7.5.2 MPS II
7.5.3 MPS IV
7.5.4 MPS VI
7.5.5 MPS III
7.6 NIEMANN-PICK DISEASE
7.6.1 TYPE C
7.6.2 TYPE B
7.6.3 TYPE A
7.7 KRABBE DISEASE
7.8 OTHERS
8 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS
8.1 OVERVIEW
8.2 IMIGLUCERASE
8.3 AGALSIDASE BETA
8.4 IDURSULFASE
8.5 ALGLUCOSIDASE ALPHA
8.6 VELAGLUCERASE
8.7 TALIGLUCERASE ALFA
8.8 LARONIDASE
8.9 AGALSIDASE ALPHA
8.1 GALSULFASE
8.11 AVALGLUCOSIDASE ALFA
8.12 OTHERS
9 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE
9.1 OVERVIEW
9.2 ENZYME REPLACEMENT THERAPY (ERT)
9.3 SUBSTRATE REDUCTION THERAPY (SRT)
9.4 CHAPERONE THERAPY
9.5 OTHERS
10 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER
10.1 OVERVIEW
10.2 MALE
10.3 FEMALE
11 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
11.4 GERIATRIC
12 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 HOSPITAL PHARMACIES
12.3 DRUGS STORES AND RETAIL PHARMACIES
12.4 ONLINE PHARMACIES
13 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 INTRAVENOUS (IV)
13.3 SUBCUTANEOUS (SC)
13.4 ORAL
13.5 OTHERS
14 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
15 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
16 SWOT ANALYSIS
17 COMPANY PROFILES
17.1 SANOFI
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 BIOMARIN
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 PFIZER INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 TAKEDA PHARMACEUTICAL COMPANY LIMITED
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 AMICUS THERAPEUTIC, INC.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CHIESI FARMACEUTICI S.P.A.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 DENALI THERAPEUTICS
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PIPELINE PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 FORGE BIOLOGICS
17.8.1 COMPANY SNAPSHOT
17.8.2 PIPELINE PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.9 JCR PHARMACEUTICALS CO., LTD.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PINELINE PRODUCT PORTFOLIO
17.9.4 PRODUCT PORTFOLIO
17.9.5 RECENT DEVELOPMENT
17.1 ORCHARD THERAPEUTICS PLC
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 PROTALIX BIOTHERAPEUTICS
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PIPELINE PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.12 REGENXBIO INC.
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PINELINE PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENT
17.13 SANGAMO THERAPEUTICS
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 SPUR THERAPEUTICS
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 ULTRAGENYX PHARMACEUTICAL INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
표 목록
TABLE 1 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 2 NORTH AMERICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 3 NORTH AMERICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 4 NORTH AMERICA FABRY DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 5 NORTH AMERICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 6 NORTH AMERICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 7 NORTH AMERICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 8 NORTH AMERICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 9 NORTH AMERICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 10 NORTH AMERICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 11 NORTH AMERICA KRABBE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 12 NORTH AMERICA OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 13 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 14 NORTH AMERICA IMIGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 15 NORTH AMERICA AGALSIDASE BETA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 16 NORTH AMERICA IDURSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 17 NORTH AMERICA ALGLUCOSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 18 NORTH AMERICA VELAGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 19 NORTH AMERICA TALIGLUCERASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 20 NORTH AMERICA LARONIDASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 21 NORTH AMERICA AGALSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 22 NORTH AMERICA GALSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 23 NORTH AMERICA AVALGLUCOSIDASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 24 NORTH AMERICA OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 25 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 26 NORTH AMERICA ENZYME REPLACEMENT THERAPY (ERT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 27 NORTH AMERICA SUBSTRATE REDUCTION THERAPY (SRT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 28 NORTH AMERICA CHAPERONE THERAPY IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 29 NORTH AMERICA OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 30 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 31 NORTH AMERICA MALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 32 NORTH AMERICA FEMALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 33 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 34 NORTH AMERICA PEDIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 35 NORTH AMERICA ADULT IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 36 NORTH AMERICA GERIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 37 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 38 NORTH AMERICA HOSPITAL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 39 NORTH AMERICA DRUGS STORES AND RETAIL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 40 NORTH AMERICA ONLINE PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 41 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 42 NORTH AMERICA INTRAVENOUS (IV) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 43 NORTH AMERICA SUBCUTANEOUS (SC) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 44 NORTH AMERICA ORAL IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 45 NORTH AMERICA OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 46 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 47 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 48 NORTH AMERICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 49 NORTH AMERICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 50 NORTH AMERICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 51 NORTH AMERICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 52 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 53 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 54 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 55 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 56 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 57 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 58 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 59 U.S. GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 60 U.S. POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 61 U.S. MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 62 U.S. NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 63 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 64 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 65 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 66 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 67 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 68 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 69 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 70 CANADA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 71 CANADA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 72 CANADA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 73 CANADA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 74 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 75 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 76 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 77 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 78 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 79 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 80 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 81 MEXICO GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 82 MEXICO POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 83 MEXICO MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 84 MEXICO NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 85 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 86 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 87 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 88 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 89 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 90 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
그림 목록
FIGURE 1 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: MULTIVARIATE MODELLING
FIGURE 7 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 SEVEN SEGMENTS COMPRISE THE NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDER TREATMENTS IS EXPECTED TO DRIVE THE NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 14 GAUCHER DISEASE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN 2025 & 2032
FIGURE 15 PESTEL ANALYSIS
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET
FIGURE 17 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2024
FIGURE 18 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2025-2032 (USD MILLION)
FIGURE 19 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, CAGR (2025-2032)
FIGURE 20 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, LIFELINE CURVE
FIGURE 21 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2024
FIGURE 22 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2025-2032 (USD MILLION)
FIGURE 23 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, CAGR (2025-2032)
FIGURE 24 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, LIFELINE CURVE
FIGURE 25 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2024
FIGURE 26 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2025-2032 (USD MILLION)
FIGURE 27 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 28 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 29 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2024
FIGURE 30 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2025-2032 (USD MILLION)
FIGURE 31 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, CAGR (2025-2032)
FIGURE 32 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 33 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2024
FIGURE 34 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2025-2032 (USD MILLION)
FIGURE 35 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, CAGR (2025-2032)
FIGURE 36 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 37 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 38 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD MILLION)
FIGURE 39 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 40 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2024
FIGURE 42 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2025-2032 (USD MILLION)
FIGURE 43 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2025-2032)
FIGURE 44 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 45 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SNAPSHOT 2024
FIGURE 46 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.