يُعدّ تعزيز التزام المريض جانبًا بالغ الأهمية من جوانب أهمية أجهزة قياس القلب عن بُعد المتنقلة (MCT). تُوفّر أجهزة MCT القابلة للارتداء حلاًّ سهلًا وغير تدخلي لمراقبة القلب بشكل مستمر. تُعزّز قدرة المرضى على دمج هذه الأجهزة بسلاسة في روتينهم اليومي التزامًا أفضل ببروتوكولات المراقبة. يضمن هذا الالتزام المُعزّز حصول مُقدّمي الرعاية الصحية على بيانات دقيقة ومتسقة على مدى فترات طويلة، مما يُسهّل الكشف المُبكر عن تشوهات القلب. تُشجّع طبيعة أجهزة MCT غير المُعطّلة المرضى على المُشاركة بفاعلية في إدارة صحتهم القلبية، مما يُؤدي في النهاية إلى اتخاذ تدابير وقائية أكثر فعالية وتدخلات في الوقت المُناسب.
يمكنك الوصول إلى التقرير الكامل على https://www.databridgemarketresearch.com/reports/canada-mobile-cardiac-telemetry-mct-market
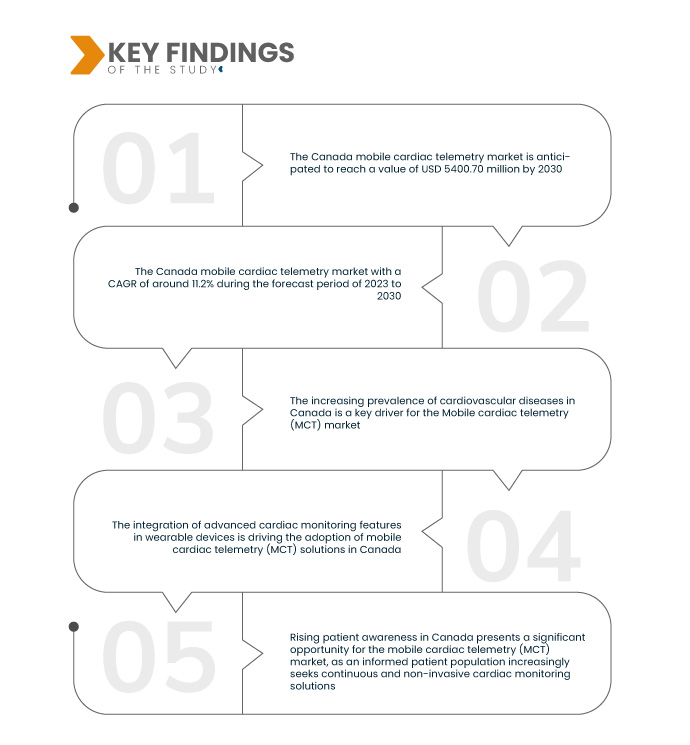
تُحلل شركة داتا بريدج لأبحاث السوق سوق أجهزة قياس القلب عن بُعد المتنقلة (MCT) في كندا ، والذي بلغ 2,310.00 مليون دولار أمريكي في عام 2022، ومن المتوقع أن يصل إلى 5,400.70 مليون دولار أمريكي بحلول عام 2030، وأن يشهد معدل نمو سنوي مركب قدره 11.2% خلال الفترة المتوقعة 2023-2030. ويُعدّ ارتفاع نسبة كبار السن في كندا، إلى جانب ازدياد احتمالية إصابة كبار السن بأمراض القلب، عاملًا رئيسيًا في نمو سوق أجهزة قياس القلب عن بُعد المتنقلة (MCT).
النتائج الرئيسية للدراسة
من المتوقع أن يؤدي تزايد السجلات الصحية الإلكترونية وأنظمة الرعاية الصحية الأوسع إلى دفع معدل نمو السوق
يُعد دمج بيانات قياس القلب عن بُعد (MCT) المتنقلة مع السجلات الصحية الإلكترونية (EHRs) وأنظمة الرعاية الصحية الأوسع نطاقًا عاملًا محوريًا في سوق قياس القلب عن بُعد (MCT) المتنقلة في كندا. يُعزز هذا التكامل التكنولوجي السلس الكفاءة التشغيلية لمقدمي الرعاية الصحية. فمن خلال تسهيل النقل الفوري لبيانات المرضى من أجهزة قياس القلب عن بُعد (MCT) إلى السجلات الصحية الإلكترونية، يحصل متخصصو الرعاية الصحية على وصول فوري إلى معلومات شاملة لمراقبة القلب. وهذا يُبسط عمليات اتخاذ القرار ويعزز نهجًا أكثر شمولية لرعاية المرضى. كما أن القدرة على دمج بيانات قياس القلب عن بُعد (MCT) في أنظمة الرعاية الصحية القائمة تُعزز منظومة رعاية صحية متماسكة ومترابطة، مما يشجع مقدمي الرعاية الصحية في كندا على تبني حلول قياس القلب عن بُعد (MCT) لتحسين نتائج المرضى وتقديم رعاية صحية قائمة على البيانات.
نطاق التقرير وتقسيم السوق
مقياس التقرير
|
تفاصيل
|
فترة التنبؤ
|
من 2023 إلى 2030
|
سنة الأساس
|
2022
|
السنوات التاريخية
|
2021 (قابلة للتخصيص حتى 2015-2020)
|
الوحدات الكمية
|
الإيرادات بالملايين من الدولارات الأمريكية، والحجم بالوحدات، والتسعير بالدولار الأمريكي
|
القطاعات المغطاة
|
النوع (متعدد القنوات، قناة واحدة)، التكنولوجيا (قائمة على الرصاص، قائمة على الرقعة، قائمة على المرونة)، الاتصال الخلوي (لاسلكي (واي فاي)، بلوتوث (BT))، المؤشرات (الذبحة الصدرية، بطء القلب، عدم انتظام ضربات القلب، احتشاء عضلة القلب، الرجفان الأذيني، انقباضات البطين المبكرة، تسرع القلب البطيني، الرجفان الأذيني المتحكم به، تصلب الشرايين، قصور القلب، وغيرها)، المستخدم النهائي (الرعاية الصحية المنزلية، المستشفيات، مرافق الاختبار التشخيصي المستقلة (IDTF)، مراكز القلب، مراكز الجراحة الخارجية، وغيرها)، قناة التوزيع (المبيعات المباشرة، وغيرها)
|
الجهات الفاعلة في السوق المغطاة
|
BIOTRONIK SE & Co. KG (ألمانيا)، Biotricity (الولايات المتحدة)، Preventice Solutions, Inc. (الولايات المتحدة)، Zio by iRhythm Technologies, Inc. (الولايات المتحدة)، Canadian Cardiac Care (كندا)، CorVitals, Inc. (الولايات المتحدة)، ACS Diagnostics (الولايات المتحدة)، Hill-Rom Services, Inc. (الولايات المتحدة)، Medicomp Inc. (الولايات المتحدة)، InfoBionic (الولايات المتحدة)، Medicalgorithmics (بولندا)، Medtronic (أيرلندا)، Medeia (فرنسا)، Laboratory Corporation of America Holdings (الولايات المتحدة)، ScottCare Corporation (الولايات المتحدة)، Abbott (الولايات المتحدة)، وBioTelemetry, Inc. (الولايات المتحدة)
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تتضمن أيضًا تحليلًا متعمقًا من الخبراء وعلم الأوبئة للمرضى وتحليل خطوط الأنابيب وتحليل التسعير والإطار التنظيمي.
|
تحليل القطاعات:
يتم تقسيم سوق قياس القلب عن بعد عبر الهاتف المحمول في كندا على أساس النوع والتكنولوجيا والاتصال الخلوي والمؤشر والمستخدم النهائي وقناة التوزيع.
- على أساس النوع، يتم تقسيم سوق قياس القلب عن بعد المحمول في كندا إلى متعدد القنوات وقناة واحدة
- على أساس التكنولوجيا، يتم تقسيم سوق قياس القلب عن بعد المحمول في كندا إلى سوق يعتمد على الرصاص، وسوق يعتمد على الرقعة، وسوق يعتمد على المرونة
- على أساس الاتصال الخلوي، يتم تقسيم سوق قياس القلب عن بعد المحمول في كندا إلى لاسلكي (WIFI) وبلوتوث (BT)
- على أساس الإشارة، يتم تقسيم سوق قياس القلب المحمول في كندا إلى الذبحة الصدرية، وبطء القلب، وعدم انتظام ضربات القلب، واحتشاء عضلة القلب، والرجفان الأذيني، وانقباضات البطين المبكرة، وتسرع القلب البطيني، والرجفان الأذيني المتحكم فيه ، وتصلب الشرايين ، وفشل القلب، وغيرها.
- على أساس المستخدم النهائي، يتم تقسيم سوق قياس القلب عن بعد المحمول في كندا إلى الرعاية الصحية المنزلية والمستشفيات ومرافق الاختبار التشخيصي المستقلة (IDTF) ومراكز القلب ومراكز الجراحة الخارجية وغيرها
- على أساس قناة التوزيع، يتم تقسيم سوق قياس القلب عن بعد عبر الهاتف المحمول في كندا إلى مبيعات مباشرة، وغيرها
اللاعبون الرئيسيون
تعترف شركة Data Bridge Market Research بالشركات التالية باعتبارها اللاعبين الرئيسيين في سوق قياس القلب عن بعد المحمول في كندا: BIOTRONIK SE & Co. KG (ألمانيا)، وBiotricity (الولايات المتحدة)، وPreventice Solutions, Inc. (الولايات المتحدة)، وZio by iRhythm Technologies, Inc. (الولايات المتحدة)، وCanadian Cardiac Care (كندا)، وCorVitals, Inc. (الولايات المتحدة)، وACS Diagnostics (الولايات المتحدة).
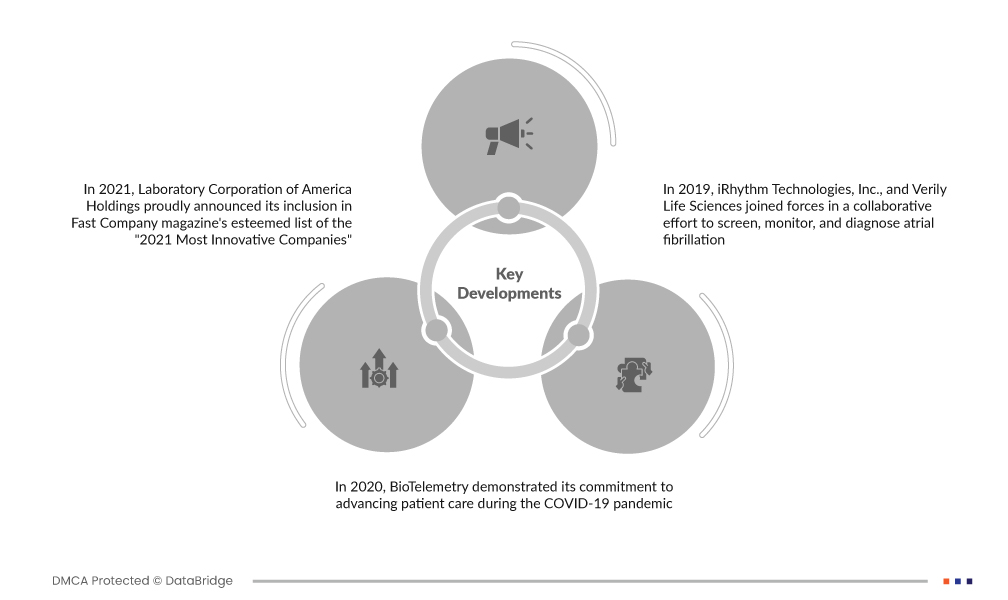
تطورات السوق
- في عام ٢٠٢١، أعلنت شركة لابوراتوري كوربوريشن أوف أمريكا هولدينغز بفخر عن إدراجها ضمن قائمة مجلة فاست كومباني المرموقة "للشركات الأكثر ابتكارًا لعام ٢٠٢١". ويُعزى هذا التكريم إلى ريادة لابوراتوري كوربوريشن المُثلى في مجالات العلوم والابتكار والرعاية الصحية، ولا سيما دورها المحوري في مكافحة جائحة كوفيد-١٩ عالميًا. وقد ساهم التقدم الذي أحرزته لابوراتوري كوربوريشن في مجال الاختبارات بشكل كبير في قدرتها على تقديم دعم أساسي للشركات حول العالم.
- في عام ٢٠٢٠، برهنت شركة BioTelemetry على التزامها بتطوير رعاية المرضى خلال جائحة كوفيد-١٩. وسّعت الشركة برنامجها لقياس القلب عن بُعد للمرضى الخارجيين (MCOT) ليشمل مرضى كوفيد-١٩ في مختلف المؤسسات بالولايات المتحدة. هدفت المبادرة إلى مراقبة إطالة فترة QT، وهو أمرٌ يُثير القلق بشأن استخدام دواء هيدروكسي كلوروكين في علاج كوفيد-١٩.
- في عام ٢٠١٩، تعاونت شركة iRhythm Technologies, Inc. مع شركة Verily Life Sciences لفحص ومراقبة وتشخيص الرجفان الأذيني. وقد استفاد هذا التعاون من تقنية الذكاء الاصطناعي من iRhythm وقدرات Verily المتقدمة في تحليل البيانات. وقد مثّلت هذه الشراكة الاستراتيجية خطوةً مهمةً في الاستفادة من أحدث التقنيات لتشخيص وإدارة الرجفان الأذيني.
لمزيد من المعلومات التفصيلية حول تقرير سوق قياس القلب عن بعد عبر الهاتف المحمول في كندا ، انقر هنا - https://www.databridgemarketresearch.com/reports/canada-mobile-cardiac-telemetry-mct-market