エジプトの注射器、自動無効化注射器、およびIVカニューラ市場は、安全性と品質に対する需要の高まりを特徴とする、ダイナミックなヘルスケアセクターです。再使用防止を目的とした自動無効化注射器は、感染症の感染予防に役立つことから注目を集めています。静脈内療法に使用されるIVカニューラも成長の可能性を秘めています。これらのトレンドは、エジプトの注射器、自動無効化注射器、およびIVカニューラ市場において、国内外のメーカーにビジネスチャンスを生み出しています。
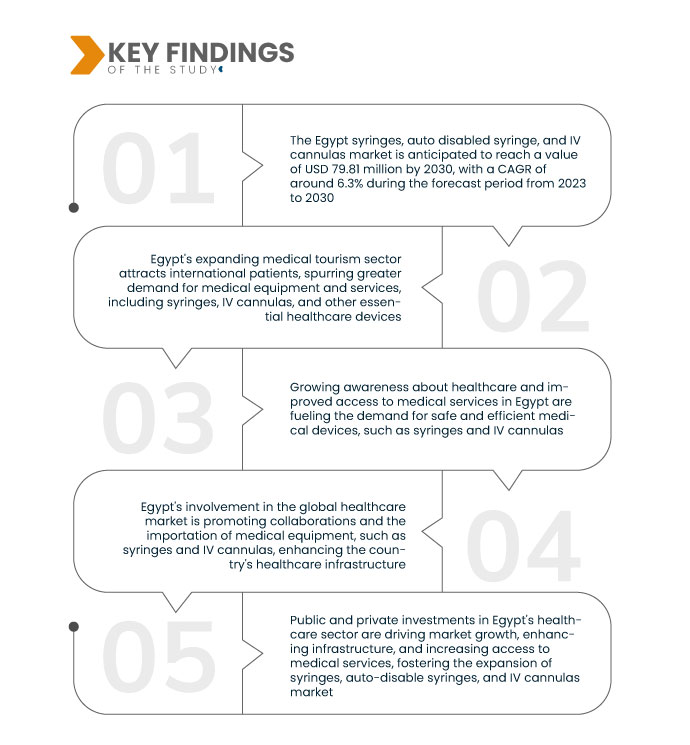
データブリッジ・マーケット・リサーチの分析によると、エジプトの注射器、自動無効化注射器、およびIVカニューラ市場は、 2023年から2030年の予測期間において5.10%の年平均成長率(CAGR)で成長し、2022年の5,361万米ドルから2030年には7,981万米ドルに達すると見込まれています。エジプトでは人口増加に伴い、注射器やIVカニューラなどの医療サービスと関連医療機器の需要が高まっています。医療を必要とする人が増えるにつれて、これらの必須ツールの需要も高まり、同国の医療機器市場の成長に貢献しています。
スタッドの主な調査結果
技術の進歩が 市場の成長率を押し上げると予想される
注射器と静脈カニューレの設計における技術進歩は、エジプトの医療機器市場において大きな製品革新をもたらしました。これらの革新には、安全機構、正確な薬剤送達のための精密工学、患者の快適性を高める材料などが含まれます。これらの改良は医療処置の有効性と安全性を高め、医療提供者がより高度で信頼性の高いツールを求める中で市場の成長を促進し、最終的には患者ケアと転帰の改善につながります。
レポートの範囲と市場セグメンテーション
レポートメトリック
|
詳細
|
予測期間
|
2023年から2030年
|
基準年
|
2022
|
歴史的な年
|
2021年(2015~2020年にカスタマイズ可能)
|
定量単位
|
売上高(百万米ドル)、販売数量(個数)、価格(米ドル)
|
対象セグメント
|
タイプ(一般用シリンジ、特殊用シリンジ、スマートシリンジ)、用途(再利用可能シリンジ、使い捨てシリンジ)、タイプ(自動無効化シリンジ、IVカニューレ)、材質(ポリプロピレンバレル、ゴムプランジャー、潤滑剤 - シリコーンオイル)、ロックシステム(バレルシールロックシステム、トッププランジャーロックシステム)、容量(0.05ML、0.1ML、0.3ML、0.5ML、1ML、その他)、ゲージサイズ(14G~16G、17G~20G、22G~26G)、IV療法(中心静脈カニューレ、ミッドラインIVカニューレ、末梢IVカニューレ)、適応症(ワクチン、感染症、炎症性および自己免疫疾患、その他)、用途(採血、ワクチン接種、薬物送達、その他)、エンドユーザー(病院、診療所、研究所、外来センター、その他)、流通チャネル(薬局、専門店、オンライン、その他)
|
対象となる市場プレーヤー
|
BD(米国)、American Equipment Company, Inc(米国)、Polymedicure(インド)、Intermedica, Inc.(ポーランド)、Denex International(インド)、Ultramedumic(カナダ)。
|
レポートで取り上げられているデータポイント
|
Data Bridge Market Research がまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、患者の疫学、パイプライン分析、価格分析、規制の枠組みも含まれています。
|
セグメント分析:
エジプトの注射器、自動無効化注射器、IV カニューレ市場は、タイプ、用途、IV 療法、材質、ロック システム、容量、ゲージ サイズ、適応症、用途、エンド ユーザー、流通チャネルに基づいて分類されています。
- タイプに基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、一般的な注射器、特殊な注射器、スマート注射器に分類されます。
- 使用方法に基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、再利用可能な注射器と使い捨て注射器に分類されます。
- IV療法に基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、中心IVカニューレ、正中線IVカニューレ、および末梢IVカニューレに分類されます。
- 材料に基づいて、エジプトの注射器、自動無効化注射器、IVカニューラ市場は、ポリプロピレンバレル、ゴムプランジャー、および潤滑剤(シリコーンオイル)に分類されます。
- ロックシステムに基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、バレルシールロックシステムとトッププランジャーロックシステムに分割されています。
- 数量に基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、0.05ML、0.1ML、0.3ML、0.5ML、1ML、その他に分類されます。
- ゲージサイズに基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、14Gから16G、17Gから20G、22Gから26Gに分類されます。
- 適応症に基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、ワクチン、感染症、炎症性および自己免疫疾患、その他に分類されます。
- アプリケーションに基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、採血、ワクチン接種、薬物送達、その他に分類されます。
- エンドユーザーに基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、病院、診療所、研究室、外来センター、その他に分類されます。
- 流通チャネルに基づいて、エジプトの注射器、自動無効化注射器、IVカニューレ市場は、薬局、専門店、オンライン、その他に分類されます。
主要プレーヤー
Data Bridge Market Research は、エジプトの注射器、自動無効化注射器、IV カニューレ市場における主要なプレーヤーとして、BD (米国)、American Equipment Company, Inc (米国)、Polymedicure (インド)、Intermedica, Inc. (ポーランド)、Denex International (インド)、Ultramedumic (カナダ) を認定しています。
市場動向
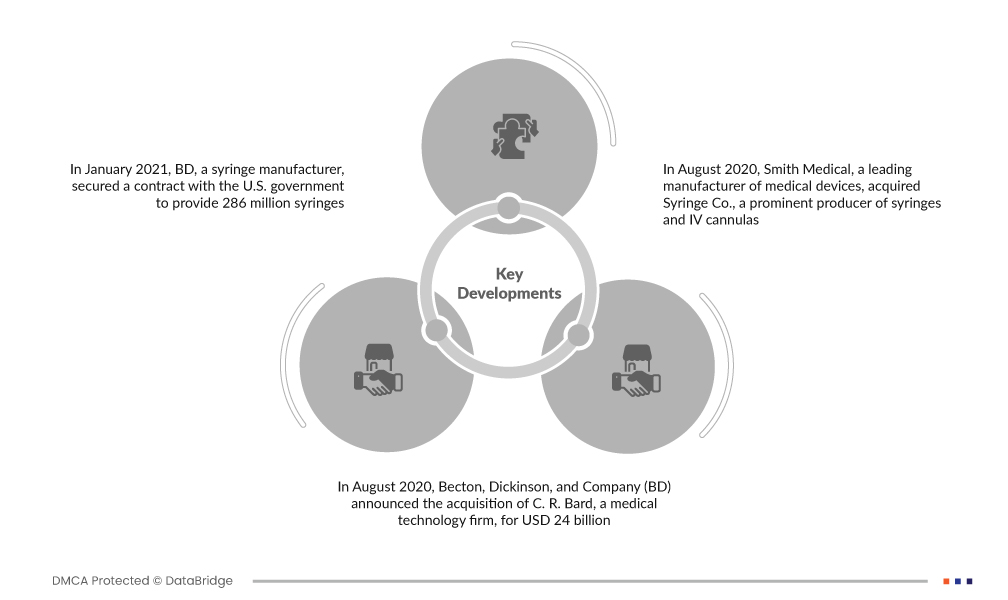
- 2021年1月、注射器メーカーのBDは、COVID-19ワクチンの配布用に、低デッドスペース注射器4,000万本を含む2億8,600万本の注射器を供給する契約を米国政府と締結しました。この戦略的提携により、BDのサプライチェーン能力が強化され、市場における効率的なワクチン供給が確保されます。
- 2020年8月、ベクトン・ディッキンソン・アンド・カンパニー(BD)は、医療技術企業であるCRバードを240億ドルで買収すると発表しました。この戦略的買収は、特に腫瘍学や外科手術などの分野における製品ポートフォリオの拡大と、患者ケアの向上に向けたイノベーションの促進を通じて、ヘルスケア業界におけるBDの地位を強化することを目的としていました。
- 2020年8月、医療機器のリーディングカンパニーであるスミス・メディカルは、注射器およびIVカニューラの主要メーカーであるシリンジ社を買収しました。この戦略的買収は、スミス・メディカルの製品ポートフォリオを拡大し、注射器、自動停止式注射器、IVカニューラ市場におけるプレゼンスを強化し、イノベーションと成長を促進することを目的としています。
エジプトの注射器、自動無効化注射器、IVカニューラ市場レポート の詳細については、ここをクリックしてください - https://www.databridgemarketresearch.com/reports/egypt-syringes-auto-disable-syringe-and-iv-cannulas-market