O mercado da pustulose palmo-plantar (PPP) aborda vários níveis de gravidade, classificados como "leve-moderado" e "moderado-grave". O diagnóstico envolve exames laboratoriais e biópsias de pele para confirmar a condição. As opções de tratamento incluem emolientes para acalmar e hidratar as zonas afetadas, esteróides tópicos para reduzir a inflamação, produtos com alcatrão de hulha para alívio dos sintomas e fototerapia , que utiliza luz UV para controlar os sintomas. Esta abordagem abrangente fornece soluções terapêuticas para os doentes com PPP, considerando a gravidade da sua condição e permitindo um atendimento personalizado.
Aceda ao relatório completo em https://www.databridgemarketresearch.com/reports/europe-palmoplantar-pustulosis-market
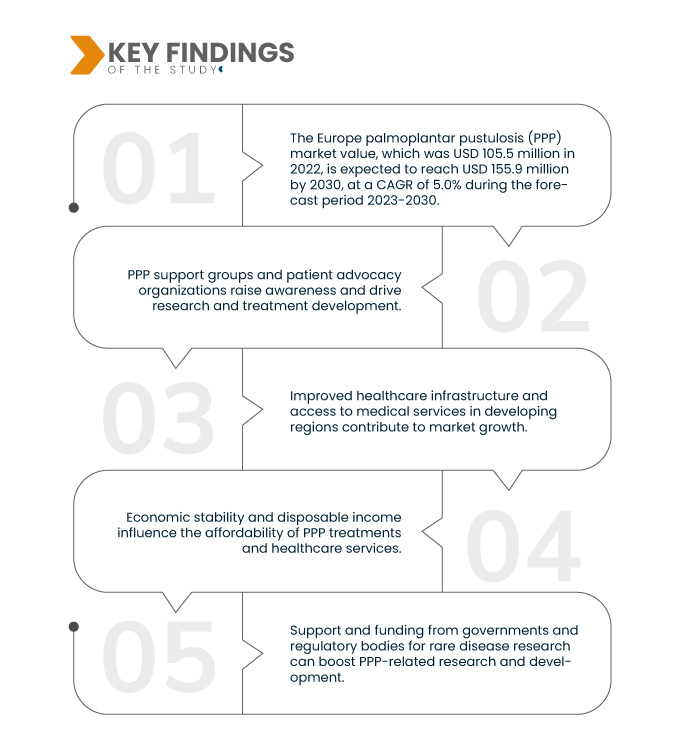
A Data Bridge Market Research analisa que o valor de mercado da pustulose palmo-plantar (PPP) na Europa , que era de 105,5 milhões de dólares em 2022, deverá atingir o valor de 155,9 milhões de dólares até 2030, com um CAGR de 5,0% durante o período previsto de 2023-2030. A crescente incidência da pustulose palmo-plantar (PPP) impulsiona o mercado ao estimular a procura de ferramentas de diagnóstico e opções de tratamento, atendendo à crescente necessidade de controlar esta condição de forma eficaz e melhorar a qualidade de vida dos doentes.
Principais conclusões do estudo
Espera-se que o envelhecimento da população impulsione a taxa de crescimento do mercado
O envelhecimento da população global impulsiona significativamente o mercado da pustulose palmo-plantar (PPP). À medida que os indivíduos envelhecem, há uma maior suscetibilidade a vários distúrbios da pele, incluindo a PPP. Esta alteração demográfica aumenta a probabilidade de ocorrência de PPP, expandindo o potencial do mercado. Os prestadores de cuidados de saúde e as empresas farmacêuticas são obrigados a atender às necessidades específicas desta população idosa, impulsionando a investigação e o desenvolvimento de tratamentos adaptados à PPP e a outras condições de pele relacionadas com a idade.
Âmbito do Relatório e Segmentação de Mercado
Métrica de Reporte
|
Detalhes
|
Período de previsão
|
2023 a 2030
|
Ano base
|
2022
|
Anos Históricos
|
2021 (personalizável para 2015-2020)
|
Unidades quantitativas
|
Receita em milhões de dólares americanos, volumes em unidades, preços em dólares americanos
|
Segmentos abrangidos
|
Tipo de doença (tipo A e tipo B), gravidade (leve-moderada e moderada-grave), tipo (diagnóstico e tratamento), tipo de população (crianças e adultos), via de administração (oral, tópica e parentérica), utilizadores finais (hospitais, cuidados domiciliários, clínicas especializadas, outros), canal de distribuição (farmácia hospitalar, farmácia on-line, farmácia de retalho)
|
Países abrangidos
|
Alemanha, França, Reino Unido, Holanda, Suíça, Bélgica, Rússia, Itália, Espanha, Turquia, Resto da Europa na Europa.
|
Participantes do mercado abrangidos
|
Novartis AG (Suíça), Lilly (EUA), F. Hoffmann-La Roche Ltd. (Suíça), Bristol-Myers Squibb Company (EUA), AstraZeneca (Reino Unido), GSK plc (Reino Unido), Merck & Co., Inc. (EUA), Amgen inc. (EUA), AnGes, Inc. (Japão), Johnson & Johnson Private Limited (EUA), AbbVie Inc. (EUA), Perrigo Company plc (Irlanda), WOCKHARDT (Índia), Bausch Health Companies Inc. (Canadá), Viatris Inc. (EUA), Amneal Pharmaceuticals LLC. (EUA), Xiromed (EUA) e Encube Ethicals Private Limited (EUA)
|
Pontos de dados abordados no relatório
|
Para além dos insights sobre os cenários de mercado, tais como o valor de mercado, a taxa de crescimento, a segmentação, a cobertura geográfica e os principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research incluem também análises aprofundadas de especialistas, epidemiologia dos doentes, análise de pipeline, análise de preços e estrutura regulamentar.
|
Análise de Segmentos:
O mercado europeu da pustulose palmo-plantar (PPP) está segmentado com base no tipo de doença, gravidade, diagnóstico, tratamento, tipo de população, via de administração, utilizador final e canal de distribuição.
- Com base no tipo de doença, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em tipo A e tipo B.
- Com base na gravidade, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em ligeira-moderada e moderada-grave.
- Com base no diagnóstico, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em testes laboratoriais e biópsia de pele .
- Com base no tratamento, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em emolientes, esteróides tópicos, alcatrão de hulha e fototerapia.
- Com base no tipo de população, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em crianças e adultos.
- Com base na via de administração, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em oral, tópico e parentérico.
- Com base no utilizador final, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em hospitais, cuidados domiciliários, clínicas especializadas e outros.
- Com base no canal de distribuição, o mercado europeu da pustulose palmo-plantar (PPP) está segmentado em farmácia hospitalar, farmácia online e farmácia de retalho.
Principais jogadores
A Data Bridge Market Research reconhece as seguintes empresas como participantes no mercado europeu de pustulose palmo-plantar (PPP): Novartis AG (Suíça), Lilly (EUA), F. Hoffmann-La Roche Ltd. (Suíça), Bristol-Myers Squibb Company (EUA), AstraZeneca (Reino Unido), GSK plc (Reino Unido), Merck & Co., Inc. (EUA).
Desenvolvimentos de mercado
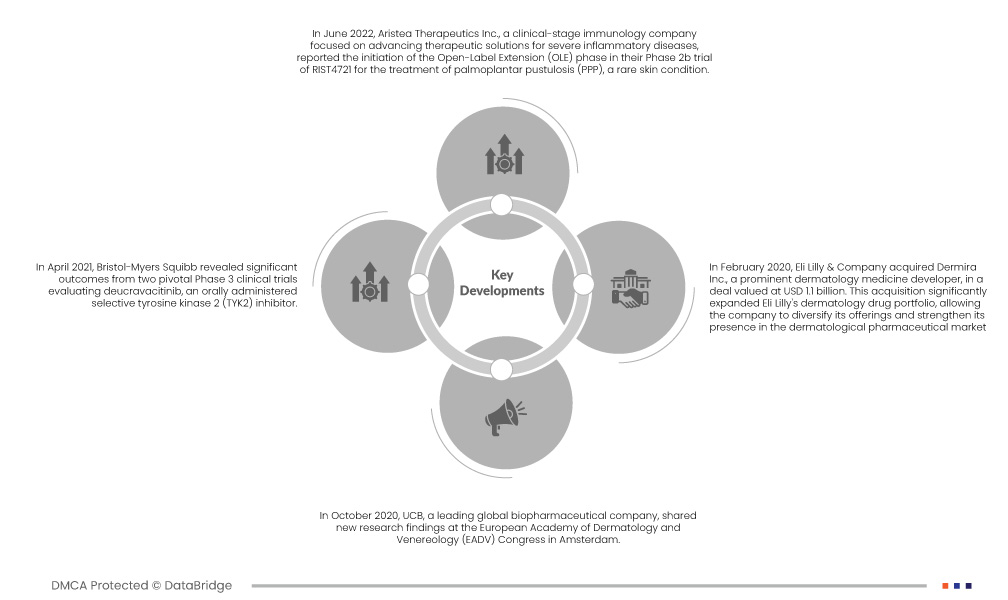
- Em junho de 2022, a Aristea Therapeutics Inc., uma empresa de imunologia em fase clínica focada no avanço das soluções terapêuticas para doenças inflamatórias graves, reportou o início da fase de extensão aberta (OLE) no seu estudo de fase 2b do RIST4721 para o tratamento da pustulose palmo-plantar (PPP), uma doença de pele rara. Administraram a primeira dose a um sujeito como parte deste estudo.
- Em abril de 2021, a Bristol-Myers Squibb revelou resultados significativos de dois ensaios clínicos essenciais de Fase 3 que avaliavam o deucravacitinib, um inibidor seletivo da tirosina quinase 2 (TYK2) administrado por via oral. Estes estudos tiveram como objetivo avaliar a eficácia do medicamento no tratamento de doentes com psoríase em placas moderada a grave , uma doença de pele autoimune comum. Os resultados realçaram o potencial do deucravacitinib como uma opção de tratamento promissora para esta condição, significando um avanço no tratamento da psoríase.
- Em outubro de 2020, a UCB, uma empresa biofarmacêutica líder a nível global, partilhou novas descobertas de investigação no Congresso da Academia Europeia de Dermatologia e Venereologia (EADV), em Amesterdão. Os dados apresentados centraram-se no inibidor experimental da IL-17A e IL-17F, bimekizumab, e no inibidor do TNF, CIMZIA (certolizumab pegol), demonstrando a sua eficácia no tratamento de doentes com psoríase em placas moderada a grave. Esta apresentação destacou o potencial destes tratamentos para abordar esta condição dermatológica desafiante.
- Em fevereiro de 2020, a Eli Lilly & Company adquiriu a Dermira Inc., uma importante empresa de desenvolvimento de medicamentos dermatológicos, num negócio avaliado em 1,1 mil milhões de dólares. Esta aquisição expandiu significativamente o portefólio de medicamentos dermatológicos da Eli Lilly, permitindo à empresa diversificar as suas ofertas e reforçar a sua presença no mercado farmacêutico dermatológico. Marcou uma mudança estratégica para fazer face à crescente procura de tratamentos dermatológicos e de inovação neste campo.
Para obter informações mais detalhadas sobre o relatório de mercado da pustulose palmoplantar (PPP) na Europa, clique aqui – https://www.databridgemarketresearch.com/reports/europe-palmoplantar-pustulosis-market