중추성 조숙증(CPP) 치료제 시장은 최근 몇 년간 빠르게 성장해 왔으며, 향후 예상 기간 동안 상당한 증가세를 보일 것으로 예상됩니다. GnRH 작용제는 내인성 GnRH의 자극 효과를 억제하고 골 발달을 촉진하여 조기 사춘기를 예방하기 때문에 CPP 치료에 사용될 수 있습니다. GnRH 치료를 통해 사춘기 시작을 지연시키면 초경이 지연됩니다. 트립토도르, 루프론 데포-페드(류프롤리드 아세테이트), 수프렐린 LA(히스트렐린 아세테이트)는 현재 중추성 조숙증(CPP) 치료제로 승인된 세 가지 치료제입니다.
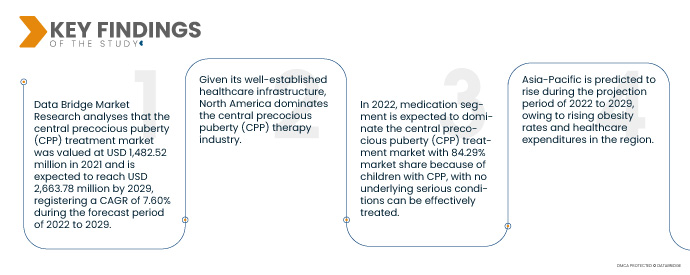
데이터 브리지 마켓 리서치(Data Bridge Market Research)에 따르면, 중추성 조숙증(CPP) 치료 시장은 2021년 14억 8,252만 달러 규모였으며, 2029년에는 26억 6,378만 달러에 도달하여 2022년부터 2029년까지 연평균 성장률 7.60%를 기록할 것으로 예상됩니다. 중추성 조숙증(CPP) 치료 시장은 과립세포종 발생률 증가, 전 세계 의료비 지출 증가, 정부의 제품 승인 지원 등의 요인으로 성장하고 있습니다. 중추성 조숙증(CPP) 치료 시장은 탄탄한 제품 파이프라인, 인지 캠페인 시작, 그리고 허가 외 의약품의 공급 확대로 인해 예측 기간 동안 확대될 것으로 예상됩니다.
증가하는 연구 개발 활동이 시장 성장률을 견인할 것으로 예상 됩니다.
중추성 조숙증 치료 시장의 성장은 R&D 활동 증가에 힘입어 촉진되고 있습니다. 이는 중추성 조숙증(CPP) 치료 산업의 새로운 성장 동력을 열어줄 것입니다. 또한, 신약 승인 및 출시 증가는 시장 성장률을 가속화할 것입니다. 더욱이, 첨단 기술 연구에 대한 투자 증가와 신흥 시장 수의 증가는 예측 기간 동안 중추성 조숙증(CPP) 치료 시장의 성장 가능성을 더욱 높여줄 것입니다.
보고서 범위 및 시장 세분화
보고서 메트릭
|
세부
|
예측 기간
|
2022년부터 2029년까지
|
기준 연도
|
2021
|
역사적인 해
|
2020 (2014-2019년으로 맞춤 설정 가능)
|
양적 단위
|
매출(백만 달러), 볼륨(단위), 가격(달러)
|
다루는 세그먼트
|
유형(약물, 수술), 진단(혈액 검사, MRI, CT 스캔, 엑스레이), 월(1개월, 2개월, 6개월, 기타), 투여 경로(비경구, 임플란트, 경구, 기타), 성별(여아, 남아), 최종 사용자(병원, 전문 클리닉, 재택 치료, 기타), 유통 채널(직접 입찰, 병원 약국, 소매 약국, 온라인 약국, 기타)
|
포함 국가
|
미국, 캐나다 및 멕시코(북미), 독일, 프랑스, 영국, 네덜란드, 스위스, 벨기에, 러시아, 이탈리아, 스페인, 터키, 유럽의 기타 유럽 국가, 중국, 일본, 인도, 한국, 싱가포르, 말레이시아, 호주, 태국, 인도네시아, 필리핀, 아시아 태평양(APAC)의 기타 아시아 태평양 국가(APAC), 사우디 아라비아, UAE, 남아프리카 공화국, 이집트, 이스라엘, 중동 및 아프리카(MEA)의 일부인 기타 중동 및 아프리카(MEA), 브라질, 아르헨티나 및 남미의 일부인 기타 남미
|
시장 참여자 포함
|
Sanofi(프랑스), Pfizer Inc.(미국), GlaxoSmithKline plc(영국), Novartis AG(스위스), AbbVie Inc.(미국), F. Hoffmann-La Roche Ltd.(스위스), Mylan NV(미국), Teva Pharmaceutical Industries Ltd.(아일랜드), Ipsen Pharma(프랑스), Arbor Pharmaceuticals(미국), Tolmar Pharmaceuticals, Inc.(미국), GP Pharm(스페인), Debiopharm(스위스), DAEWOONG PHARMACEUTICAL CO.,LTD(한국), Sun Pharmaceutical Industries Ltd.(인도), Takeda Pharmaceutical Company Limited(일본), Endo International plc(아일랜드), AstraZeneca(영국), Johnson & Johnson Private Limited(미국)
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research 팀이 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 시장 부문, 지리적 적용 범위, 시장 참여자 및 시장 시나리오와 같은 시장 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석 및 규제 프레임워크가 포함되어 있습니다.
|
세그먼트 분석:
글로벌 중추성 조숙성 사춘기(CPP) 치료 시장은 유형, 월, 투여 경로, 성별, 최종 사용자 및 유통 채널을 기준으로 6가지 주요 세그먼트로 분류됩니다.
- 중추성 조숙증(CPP) 치료 시장은 약물 치료와 수술 치료로 구분됩니다. 약물 치료는 중추성 조숙증(CPP) 치료 시장의 83.29%를 차지하며 시장을 주도할 것으로 예상됩니다. 기저 질환이 없는 CPP 아동은 약물 치료로 효과적으로 치료할 수 있으며, 약물 치료가 CPP 관리 및 치료에 있어 최우선 선택이기 때문입니다.
- 월 기준으로 중추성 조숙성 사춘기(CPP) 치료 시장은 1개월, 3개월, 6개월 및 기타로 구분됩니다. 1개월 세그먼트는 38.47%의 시장 점유율로 중추성 조숙성 사춘기(CPP) 치료 시장을 지배할 것으로 예상됩니다. 그 이유는 CPP 진단을 받은 아동에게 치료 효과를 모니터링하기 위해 1개월 동안 초기 치료를 실시했기 때문입니다.
- 투여 경로를 기준으로 중추성 조숙증(CPP) 치료 시장은 비경구, 경구, 임플란트 및 기타로 구분됩니다. 비경구 시장은 GnRH의 지속 방출 제형과 지속적인 장기 투여(주사제)가 필요한 CPP 치료에 사용되는 약물 덕분에 56.13%의 시장 점유율을 기록하며 중추성 조숙증(CPP) 치료 시장을 주도할 것으로 예상됩니다.
- 성별에 따라 중추성 조숙성 사춘기(CPP) 치료 시장은 여아와 남아로 구분됩니다. 여아 부문은 CPP가 여아에 비해 남아에게 덜 흔하고 남성 인구에 비해 여성 비율이 높기 때문에 중추성 조숙성 사춘기(CPP) 치료 시장을 72.41%의 시장 점유율로 지배할 것으로 예상됩니다.
- 최종 사용자 기준으로 중추성 조숙증(CPP) 치료 시장은 병원, 전문 클리닉, 홈케어 등으로 세분화됩니다. 2022년에는 병원 부문이 중추성 조숙증(CPP) 치료 시장을 52.58%의 점유율로 주도할 것으로 예상됩니다. 병원이 CPP 진단 및 치료의 선두 주자이기 때문입니다.
병원 센터 부문은 중추성 조숙성 사춘기(CPP) 치료 시장의 최종 사용자 부문을 지배할 것입니다.
병원 센터 부문은 시장 내 주요 최종 사용자 부문으로 부상할 것입니다. 이는 특히 개발도상국에서 병원 수가 증가하고 있기 때문입니다. 또한, 전 세계적으로 연구 개발 서비스가 성장하고 확대됨에 따라 이 부문의 성장은 더욱 가속화될 것입니다.
- 유통 채널을 기준으로, 중추성 조숙성 사춘기(CPP) 치료 시장은 직접 입찰, 병원 약국, 소매 약국, 온라인 약국 및 기타로 세분화됩니다. 직접 입찰 부문은 CPP 치료에 사용되는 약물을 합리적인 가격으로 직접 입찰을 통해 조달할 수 있고 환자가 장기간 치료를 받을 수 있기 때문에 48.72%의 시장 점유율로 중추성 조숙성 사춘기(CPP) 치료 시장을 지배할 것으로 예상됩니다.
직접 입찰 부문은 중추성 조숙증(CPP) 치료 시장의 유통 채널 부문을 지배할 것입니다.
직접 입찰 부문은 약 50%의 시장 점유율을 기록하며 유통 채널에서 가장 큰 비중을 차지하는 부문으로 부상할 것입니다. 이는 특히 개발도상국에서 인프라 개발 활동이 증가하고 있기 때문입니다. 또한, 전 세계적으로 헬스케어 산업의 성장과 확장은 이 부문의 성장을 더욱 가속화할 것입니다.
주요 플레이어
Data Bridge Market Research에서는 다음 회사를 주요 시장 주체로 인식합니다. Sanofi(프랑스), Pfizer Inc.(미국), GlaxoSmithKline plc(영국), Novartis AG(스위스), AbbVie Inc.(미국), F. Hoffmann-La Roche Ltd.(스위스), Mylan NV(미국), Teva Pharmaceutical Industries Ltd.(아일랜드), Ipsen Pharma(프랑스), Arbor Pharmaceuticals(미국), Tolmar Pharmaceuticals, Inc.(미국), GP Pharm(스페인), Debiopharm(스위스), DAEWOONG PHARMACEUTICAL CO.,LTD(한국), Sun Pharmaceutical Industries Ltd.(인도), Takeda Pharmaceutical Company Limited(일본), Endo International plc(아일랜드), AstraZeneca(영국), Johnson & Johnson Private Limited(미국).
시장 개발
- 2019년 1월, 다케다제약은 중추성 조숙증(CPP)과 같은 희귀 질환 치료제를 생산하는 샤이어(Shire plc) 인수를 완료했습니다. 이번 인수를 통해 중추성 조숙증(CPP) 치료제를 포함한 다케다제약의 제품 포트폴리오가 더욱 강화되었습니다.
- 2019년 6월, 입센 파마(Ipsen Pharma)는 데비오팜(Debiopharm)과의 전략적 협력 관계를 15년 더 연장했습니다. 데카펩틸(트립토렐린)은 중추성 조숙증(CPP) 소아 치료에 사용됩니다. 이 전략적 제휴를 통해 입센 파마는 환자들에게 지속적인 제품 공급을 유지할 수 있었습니다.
지역 분석
지리적으로 시장 보고서에서 다루는 국가는 북미의 미국, 캐나다 및 멕시코, 유럽의 독일, 프랑스, 영국, 네덜란드, 스위스, 벨기에, 러시아, 이탈리아, 스페인, 터키, 유럽의 기타 유럽, 중국, 일본, 인도, 한국, 싱가포르, 말레이시아, 호주, 태국, 인도네시아, 필리핀, 아시아 태평양(APAC)의 기타 아시아 태평양(APAC), 사우디 아라비아, UAE, 남아프리카 공화국, 이집트, 이스라엘, 중동 및 아프리카(MEA)의 일부인 기타 중동 및 아프리카(MEA), 남미의 일부인 기타 남미입니다.
Data Bridge Market Research 분석에 따르면:
북미는 2022년부터 2029년까지의 예측 기간 동안 중추성 조숙증(CPP) 치료 시장 에서 가장 큰 비중을 차지하는 지역입니다.
북미는 탄탄한 의료 인프라를 바탕으로 성조숙증(CPP) 치료 산업의 중추를 장악하고 있습니다. 더욱이, 주요 기업들이 신기술에 더욱 집중함에 따라 이 분야의 시장 성장률은 더욱 가속화될 것입니다.
아시아 태평양 지역은 2022년부터 2029년까지의 예측 기간 동안 중추성 조숙증(CPP) 치료 시장 에서 가장 빠르게 성장하는 지역으로 추정됩니다.
아시아 태평양 지역은 2022년에서 2029년까지 비만율 증가와 의료비 지출 증가로 인해 성장할 것으로 예측됩니다.
중추성 조숙성 사춘기(CPP) 치료 시장 보고서 에 대한 자세한 내용은 여기를 클릭하세요. - https://www.databridgemarketresearch.com/reports/global-central-precocious-puberty-cpp-treatment-market