According to the WHO's 2020, colorectal cancer is the third most common cancer type worldwide; in 2020, almost 2 million cases were diagnosed. Colorectal cancer is the second most common cause of cancer death, leading to almost 1 million deaths annually. This is despite effective techniques that could reduce the number of deaths from this disease exists. Therefore, the global advanced colorectal cancer diagnostics market is estimated to increase rapidly shortly.
Access Full Report at @ https://www.databridgemarketresearch.com/reports/global-colorectal-cancer-diagnostics-market
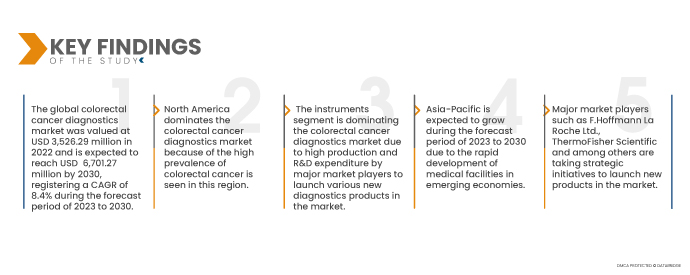
Data Bridge Market Research analyses that the Colorectal Cancer Diagnostics Market is expected to grow at a CAGR of 8.4% in the forecast period of 2023 to 2030, and is expected to reach USD 6,701.27 million by 2030. The instruments are projected to propel the market's growth as they are widely used for diagnosis in various regions, and more advanced technologies are available. Especially these instruments have a broad range of applications for research and clinical purposes, including cancer diagnosis, chromosome identification, and protein expression.
Growing Prevalence of Colorectal Cancer
Colorectal cancer is sometimes called colon cancer, which is a term that combines colon cancer and rectal cancer, which begins in the rectum. Colon cancer typically affects older adults, though it can happen at any age. It usually begins as small, noncancerous (benign) clumps of cells called polyps forming inside the colon. Over time some of these polyps can become colon cancers.
People affected with colorectal cancer can see the symptoms such as a persistent change in bowel habits, including diarrhea or constipation or a change in the consistency of the stool, rectal bleeding or blood in the stool, persistent abdominal discomfort, such as cramps, gas or pain, a feeling that the bowel doesn't empty, weakness or fatigue, and unexplained weight loss.
Global Colorectal Cancer Diagnostics Market Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2020-2016)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
By Product Type (Instruments and Consumables & Accessories), Test Type (Stool Examination, Blood Test, Imaging Test, Tumor Markers, Biopsy, and Others), Cancer Stages (Stage 0, Stage I, Stage II, Stage III, and Stage IV), Cancer Type (Adenocarcinoma, Colorectal Lymphoma, Gastrointestinal Stromal Tumors, Carcinoid Tumors, and Others), Age Group (Geriatric, Adults, and Pediatric), End User (Hospitals, Diagnostic Centers, Cancer Research Centers, Ambulatory Surgical Centers, Academic Institutes, and Others)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, France, U.K, Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium, Rest of Europe, China, Japan, India, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, and Rest of Middle East and Africa.
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd (Switzerland), Koninklijke Philips N.V. (Netherland), Thermo Fisher Scientific Inc. (U.S.), Illumina, Inc. (U.S.), Quest Diagnostics Incorporated (U.S.), Canon Medical Systems Corporation (Japan), Bio-Rad Laboratories, Inc. (U.S.), Merck KGaA (Germany), FUJIFILM Corporation (Japan), Agilent Technologies, Inc. (U.S.), BD (U.S.), General Electric Company (U.S.), Siemens Healthcare GmbH (Germany), Abbott (U.S.), Neusoft Corporation (China), BioFire Diagnostics (U.S.), Myriad Genetics Inc. (U.S.), QIAGEN (U.S.), Hologic Inc. (U.S.), Time Medical Holding. (Hongkong), FONAR Corp. (U.S.), PlexBio. (Taiwan), SternMed GmbH. (Germany), MinFound Medical Systems Co., Ltd (China), Medonica Co. LTD (Korea), and Beijing O&D Biotech Co., Ltd. (China).
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Global Colorectal Cancer Diagnostics Market Segment Analysis:
The colorectal cancer diagnostics market is segmented based on product type, test type, cancer stages, cancer type, age group, and end user.
On the basis of product type, the advanced colorectal cancer diagnostics market is classified into instruments and consumables & accessories.
In 2023, the instruments segment of product type segment is anticipated to dominate the colorectal cancer diagnostics market
In 2023, the instruments segment of this market is expected to dominate the colorectal cancer diagnostics market owing to the increasing utilization of various instrument types as biopsies and imaging for colorectal cancer diagnosis.
- On the basis of test type, the global colorectal cancer diagnostics market is segmented into stool examination, imaging test, biopsy, blood test, tumor markers, and others. In 2023, stool examination is expected to dominate the market with 39.41% market share due to the easy collection of samples for test and the wide use of this type of tests.
- On the basis of cancer stages, the global colorectal cancer diagnostics market is segmented into stage 0, stage I, stage II, stage III, and stage IV. In 2023, the stage iii segment is expected to dominate the market with a 41.33% market share due to the increasing awareness of the early diagnosis of colorectal cancer is driving the growth of this segment in the market.
- On the basis of cancer type, the global colorectal cancer diagnostics market is segmented into adenocarcinoma, colorectal lymphoma, gastrointestinal stromal tumors, carcinoid tumors, and others. In 2023, the adenocarcinoma segment is expected to dominate the market with a 62.00% market share due to rising diagnostic product approval for colorectal cancer and increasing healthcare spending.
- On the basis of age group, the global colorectal cancer diagnostics market is segmented into pediatric, adult, and geriatric. In 2023, the geriatric segment is expected to dominate the market with 46.95% market share due to awareness about regular healthcare checkups, upcoming diagnostic centers, and advancements in diagnostic methods for colorectal cancer and technological developments are on the urge.
- On the basis of end user, the global colorectal cancer diagnostics market is segmented into hospitals, diagnostic centers, cancer research centers, ambulatory surgical centers, academic institutes, and others. In 2023 the hospitals segment is expected to dominate the market with 30.77% market share due to the increasing adoption of advanced products and rising healthcare concern of the patient over the past few years.
F. Hoffmann-La Roche Ltd, Koninklijke Philips N.V. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Illumina (U.S.), Quest Diagnostics Incorporated (U.S.), Canon Medical Systems Corporation (Japan), Bio-Rad Laboratories Inc.(U.S.), Merck KGaA (Germany), FUJIFILM Corporation (Japan), Agilent Technologies Inc. (U.S.), BD (U.S.), General Electric Company (U.S.), Siemens Healthcare GmbH (Germany), Abbott (U.S.), BioFire Diagnostics (U.S.), Myriad Genetics Inc. (U.S.), QIAGEN (U.S.), Time Medical Holding. (U.S.), FONAR Corp. (U.S.), and MinFound Medical Systems Co., Ltd (China) are developing colorectal cancer diagnostics for hospitals, diagnostic centers, cancer research centers, ambulatory surgical centers, academic institutes, and others.
In 2023, the instruments segment is projected to hold the largest share of product type segment in the colorectal cancer diagnostics market
In 2023, the instruments segment holds the top market share market will dominate the colorectal cancer diagnostics market owing to the increasing utilization of various instrument types as biopsies and imaging for colorectal cancer diagnosis. The instruments segment is expected to reach the highest CAGR of 9.3% in 2023-2030.
- On the basis of end user, the global colorectal cancer diagnostics market is segmented into hospitals, diagnostic centers, cancer research centers, ambulatory surgical centers, academic institutes, and others. The hospitals segment is expected to dominate the market with a 46.53% market share due to the increasing adoption of advanced products as well as rising healthcare concern of the patient over the past few years.
Major Players
Data Bridge Market Research recognizes the following companies as the major in colorectal cancer diagnostics market are F. Hoffmann-La Roche Ltd (Switzerland), Koninklijke Philips N.V. (Netherland), Thermo Fisher Scientific Inc. (U.S.), Illumina, Inc. (U.S.) , Quest Diagnostics Incorporated (U.S.), Canon Medical Systems Corporation (Japan), Bio-Rad Laboratories, Inc. (U.S.), Merck KGaA (Germany), FUJIFILM Corporation (Japan), Agilent Technologies, Inc. (U.S.), BD (U.S.), General Electric Company (U.S.), Siemens Healthcare GmbH (Germany), Abbott (U.S.), Neusoft Corporation (China), BioFire Diagnostics (U.S.), Myriad Genetics Inc. (U.S.), QIAGEN (U.S.), Hologic (U.S.), Time Medical Holding. (Hongkong), FONAR Corp. (U.S.), PlexBio. (Taiwan), SternMed GmbH. (Germany), MinFound Medical Systems Co., Ltd (China), Medonica Co. LTD (Korea), and Beijing O&D Biotech Co., Ltd. (China).
Market Development
- In November 2022, Abbott was recognized by the Consumer Technology Association (CTA) with three CES 2023 Innovation Awards for its life-changing technologies advancing the health tech industry and improving people's lives worldwide. The CTA is the organization behind the Consumer Electronics Show (CES), the most influential technology event in the world. This has helped the company to increase its global presence in the market.
- In October 2022, Koninklijke Philips N.V. announced the imaging and simulation of head and neck cancer radiation had advanced thanks to two significant developments in MR-only workflows. In the company's artificial intelligence-enabled MRCAT radiation application, soft tissue tumors of the head and neck and those of the brain, pelvis, and prostate can be treated using MRI as the primary imaging modality for planning. The device has been given FDA 510(k) clearance and is offered for sale in the United States. This helps the company to increase its global presence of the company as well as helps in revenue expansion.
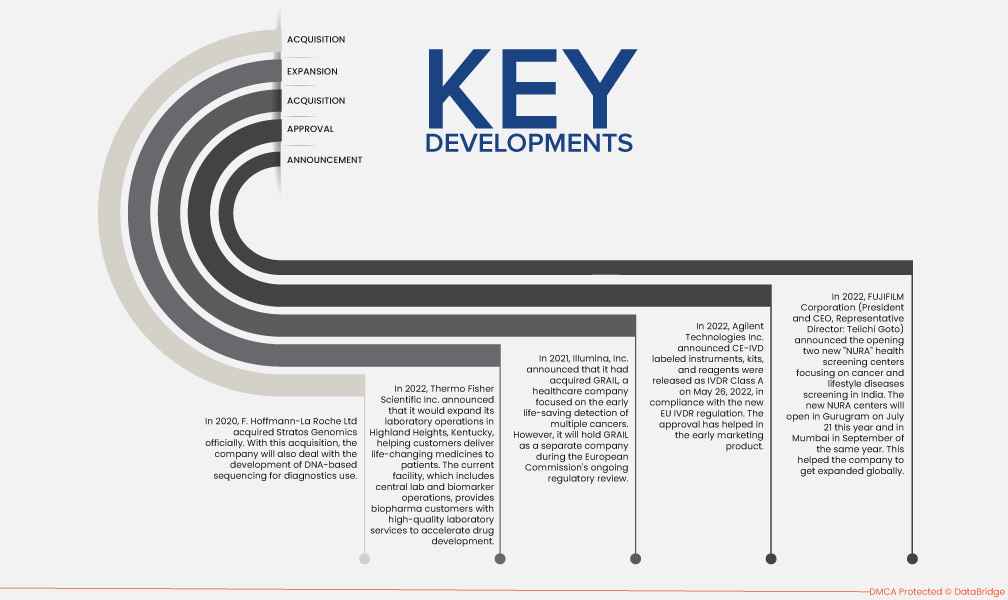
- In October 2022, Thermo Fisher Scientific Inc. announced that it would expand its laboratory operations in Highland Heights, Kentucky, helping customers deliver life-changing medicines to patients. The current facility, which includes central lab and biomarker operations, provides biopharma customers with high-quality laboratory services to accelerate drug development. This has helped the company to expand its clinical diagnostics business across various regions in the world and helped to increase the global presence in the market.
- In August 2022, Bio-Rad Laboratories Inc. completed its acquisition of Curiosity Diagnostics, a Poland-based developer of innovative technology solutions for the medical diagnostic and healthcare markets. This has helped the company to increase its product portfolio and global presence in the market.
- In May 2020, F. Hoffmann-La Roche Ltd acquired Stratos Genomics officially. With this acquisition, the company will also deal with the development of DNA-based sequencing for diagnostics use. This has enhanced the healthcare diagnosis segment of the company, thus leading to more revenue generation for the company.
Regional Analysis
Geographically, the countries covered in the colorectal cancer diagnostics market report are U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Russia, Turkey, Netherlands, Belgium, Switzerland, rest of Europe in Europe, China, India, Japan, South Korea, Thailand, Singapore, Indonesia, Malaysia, Philippines, Australia, Vietnam, rest of Asia-Pacific, Brazil, Argentina, rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and rest of Middle East and Africa.
As per Data Bridge Market Research analysis:
North America is the dominant region in colorectal cancer diagnostics market during the forecast period 2023-2030
In 2022, North America dominated the colorectal cancer diagnostics market owing to the higher level of investments by U.S. manufacturers and the increasing prevalence of cancer diagnostics in the U.S. North America will continue to dominate the colorectal cancer diagnostics market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the growing adoption of advanced technology and the launch of new products in this region. Additionally, the increasing number of cancer cases and the rising geriatric population are expected further to enhance the market's growth rate in this region.
Asia-Pacific is estimated to be the fastest-growing region in the colorectal cancer diagnostics market the forecast period 2023-2030
Asia-Pacific is expected to grow during the forecast period due to the presence of major market players and the rapid development of medical facilities in emerging economies in this region. In addition, rising healthcare expenditure and increasing per-capita income are expected to propel the market's growth rate in this region.
Global Colorectal Cancer Diagnostics Market COVID-19 Impact Analysis
The outbreak of COVID-19 largely impacted the healthcare industry. The advanced wound care market was, however, adversely affected by it. The imposition of the lockdown and social distancing restrictions by the government to curb the COVID-19 pandemic outbreak led to the emergence of various challenges, such as the denied diagnostics centers, canceled or postponed diagnostic treatment, stifling business growth, suspensions of new developments, and even increasing cases in COVID-19 medical care providers, further limiting the industry's expansion. As the admission of COVID-19 patients in hospitals increased, numerous cancer diagnostics were canceled or postponed to reserve hospital beds and patient care staff for COVID-19 patient care. Developing and implementing contingency plans is crucial for business operations and key imported raw materials.
On the brighter side, there has been a decline in COVID-19 patients worldwide, which will result in increased cancer care services. Moreover, the restrictions and measures are likely to relax, which will help the market to witness a slight increment, as the manufacturers focus on various developments and innovations, market trends, and other expansion strategies. Thus, the colorectal cancer diagnostics market will grow accelerated post-COVID-19.
For more detailed information about the colorectal cancer diagnostics market report, click here – https://www.databridgemarketresearch.com/reports/global-colorectal-cancer-diagnostics-market