Global Colorectal Cancer Diagnostics Market
Market Size in USD Billion
CAGR :
% 
 USD
4.14 Billion
USD
7.89 Billion
2024
2032
USD
4.14 Billion
USD
7.89 Billion
2024
2032
| 2025 –2032 | |
| USD 4.14 Billion | |
| USD 7.89 Billion | |
|
|
|
|
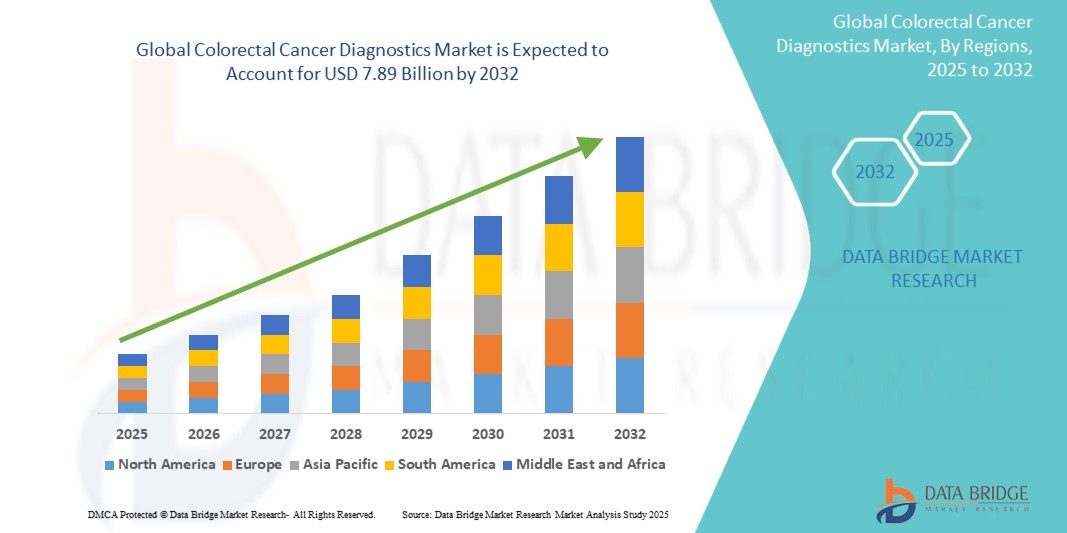
Colorectal Cancer Diagnostics Market Size
- The global colorectal cancer diagnostics market size was valued at USD 4.14 billion in 2024 and is expected to reach USD 7.89 billion by 2032, at a CAGR of 8.40% during the forecast period
- The market growth is largely fueled by the increasing prevalence of colorectal cancer, rising awareness about early detection, and technological advancements in diagnostic tools, leading to improved accuracy and faster turnaround times in both clinical and research settings
- Furthermore, growing demand for minimally invasive procedures, precision medicine, and personalized treatment strategies is driving the adoption of advanced colorectal cancer diagnostics solutions, thereby significantly boosting the industry's growth
Colorectal Cancer Diagnostics Market Analysis
- The global colorectal cancer diagnostics market is witnessing strong growth due to rising colorectal cancer prevalence, increasing awareness about early detection, and continuous advancements in diagnostic technologies such as imaging tests, liquid biopsies, and molecular diagnostics. Early diagnosis and improved patient outcomes are driving the adoption of advanced screening solutions across healthcare facilities worldwide
- The escalating demand for colorectal cancer diagnostics is primarily fueled by technological innovations, government screening initiatives, and the growing elderly population, which is more susceptible to colorectal cancer. Non-invasive and accurate diagnostic solutions are increasingly preferred by both clinicians and patients, contributing to market expansion
- North America dominated the colorectal cancer diagnostics market with the largest revenue share of 42.5% in 2024, attributed to well-established healthcare infrastructure, high adoption of advanced screening technologies, and significant investments by key market players. The U.S. has experienced substantial growth in colorectal cancer diagnostic implementations in hospitals, diagnostic imaging centers, and multi-specialty clinics, driven by artificial intelligence -assisted imaging, liquid biopsy innovations, and early detection programs
- Asia-Pacific is expected to be the fastest-growing region in the colorectal cancer diagnostics market during the forecast period, with a projected CAGR of 7.8%, owing to increasing urbanization, rising disposable incomes, expanding healthcare infrastructure, and growing awareness about cancer screening programs. Countries such as China, India, and Japan are witnessing rapid adoption of advanced diagnostics to meet rising demand
- The instruments segment dominated the largest market revenue share of 46.5% in 2024, driven by the increasing adoption of advanced diagnostic technologies across hospitals, cancer research centers, and specialized clinics
Report Scope and Colorectal Cancer Diagnostics Market Segmentation
|
Attributes |
Colorectal Cancer Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Colorectal Cancer Diagnostics Market Trends
Advancements in Early Detection and Non-Invasive Testing
- A significant and accelerating trend in the global colorectal cancer diagnostics market is the growing adoption of advanced early detection technologies, including non-invasive stool-based tests, liquid biopsies, and next-generation sequencing (NGS). These innovations are enhancing diagnostic accuracy, patient compliance, and timely intervention
- For instance, non-invasive fecal immunochemical tests (FIT) and multi-target stool DNA tests are increasingly preferred for population-level screening, enabling earlier detection of colorectal cancer and precancerous lesions. Early-stage diagnosis improves survival rates and reduces treatment complexity, which is driving strong adoption among healthcare providers
- The integration of molecular diagnostics and precision medicine is further advancing colorectal cancer detection. NGS panels and biomarker-based assays allow for more personalized risk assessment, aiding clinicians in identifying patients at high risk and recommending tailored screening schedules
- The rising awareness of colorectal cancer risks, coupled with government screening programs and insurance coverage, is expanding the market. Hospitals, specialty clinics, and diagnostic laboratories are increasingly incorporating these modern diagnostic solutions to improve patient outcomes
- Furthermore, technological advancements in imaging, such as high-definition colonoscopy and AI-assisted endoscopic analysis, are improving lesion detection rates and minimizing missed diagnoses, thereby strengthening confidence in clinical decision-making
- Overall, the trend toward more accurate, non-invasive, and patient-friendly diagnostics is reshaping expectations for colorectal cancer screening and management. The growing emphasis on early detection, personalized testing, and accessible diagnostics continues to drive rapid market growth across both developed and emerging regions
Colorectal Cancer Diagnostics Market Dynamics
Driver
Growing Need Due to Rising Prevalence and Awareness of Colorectal Cancer
- The rising incidence of colorectal cancer worldwide, combined with increasing awareness about the critical importance of early detection, is driving the demand for advanced diagnostic solutions. This trend reflects the growing focus of healthcare systems on preventive care and population-wide screening programs
- For instance, in 2024, several leading diagnostic companies introduced next-generation stool DNA tests and high-sensitivity molecular panels that improve early detection accuracy and reduce false negatives. Such technological advancements are expected to significantly boost the Colorectal Cancer Diagnostics market during the forecast period
- As both patients and healthcare providers recognize the clinical benefits of early diagnosis, non-invasive diagnostic options—such as liquid biopsies and fecal immunochemical tests (FIT)—are gaining traction due to higher patient compliance and less discomfort compared to traditional invasive procedures such as colonoscopy
- The expansion of government and private screening initiatives is making colorectal cancer diagnostics increasingly integral to national health strategies. Public awareness campaigns, insurance coverage, and routine hospital screening programs are reinforcing adoption in both urban and semi-urban regions
- Moreover, the integration of molecular diagnostics with personalized treatment planning allows physicians to tailor therapy based on patient-specific risk profiles, further enhancing the clinical relevance of these advanced diagnostic tools
- The emphasis on preventive healthcare, coupled with technological innovation and rising patient demand, continues to create strong growth momentum across hospitals, specialty clinics, and diagnostic laboratories globally
Restraint/Challenge
High Costs and Limited Access in Emerging Markets
- The relatively high cost of advanced diagnostic procedures, such as next-generation sequencing panels and multi-target stool DNA tests, remains a key barrier to wider market penetration, particularly in price-sensitive emerging regions
- Limited availability of specialized diagnostic laboratories and trained personnel in rural or underdeveloped areas further restricts access, creating disparities in early detection and timely treatment
- Overcoming these challenges requires strategic initiatives such as government-subsidized programs, partnerships with local diagnostic centers, and the development of cost-effective testing solutions to enhance accessibility
- Although adoption is increasing in urban centers, broader market growth in developing countries will depend on investments in healthcare infrastructure, awareness campaigns, and the provision of affordable testing solutions
- Innovations such as home-based sample collection kits and portable point-of-care diagnostic tools are expected to address accessibility challenges, supporting sustained expansion of the Colorectal Cancer Diagnostics market globally
Colorectal Cancer Diagnostics Market Scope
The market is segmented on the basis of product type, test type, cancer stages, cancer type, age group, and end-user.
By Product Type
On the basis of product type, the colorectal cancer diagnostics market is segmented into instruments and consumables & accessories. The instruments segment dominated the largest market revenue share of 46.5% in 2024, driven by the increasing adoption of advanced diagnostic technologies across hospitals, cancer research centers, and specialized clinics. Sophisticated instruments, including high-definition colonoscopes, endoscopic imaging systems, automated analyzers, and molecular diagnostic platforms, play a pivotal role in the accurate detection, staging, and continuous monitoring of colorectal cancer. These tools not only enhance diagnostic precision but also support timely clinical decision-making, ultimately improving patient outcomes and survival rates.
The consumables & accessories segment is projected to witness the fastest CAGR of 12.3% from 2025 to 2032, fueled by the rising demand for disposable test kits, reagents, specimen collection devices, and other essential ancillary products. These consumables enable contamination-free sample handling, streamline laboratory workflows, and facilitate efficient large-scale population screening programs. Growing awareness about preventive diagnostics, combined with increasing government and private initiatives for early cancer detection, further accelerates the adoption of consumables and accessories in colorectal cancer diagnostics worldwide.
• By Test Type
On the basis of test type, the colorectal cancer diagnostics market is segmented into stool examination, blood test, imaging test, tumor markers, biopsy, and others. Stool examination held the largest revenue share of 41.2% in 2024, primarily due to its non-invasive nature, ease of use, affordability, and suitability for large-scale population screening. This method allows early detection of colorectal abnormalities without requiring complex equipment or procedures, making it highly accessible for both urban and rural healthcare settings. Its widespread adoption is further supported by established clinical guidelines recommending routine stool-based screening for adults above 45 years.
The blood test segment is expected to register the fastest CAGR of 13.1% from 2025 to 2032, propelled by advancements in liquid biopsy technologies, molecular diagnostics, and highly sensitive biomarker detection. Blood-based diagnostics are increasingly preferred for their ability to detect cancer at very early stages, monitor disease progression in real time, and guide personalized treatment strategies. Growing awareness among clinicians and patients, along with the integration of blood tests into regular screening programs, is accelerating the adoption of this segment globally.
• By Cancer Stages
On the basis of cancer stages, the colorectal cancer diagnostics market is segmented into Stage 0, Stage I, Stage II, Stage III, and Stage IV. Stage II colorectal cancer accounted for the largest market revenue share of 38.7% in 2024, highlighting the clinical significance of identifying cancer before it progresses to advanced stages where treatment becomes more complex and survival rates drop. Detecting Stage II cancer allows for effective interventions such as surgery and adjuvant therapy, significantly improving patient outcomes and quality of life.
Stage III is expected to witness the fastest CAGR of 11.8% from 2025 to 2032, fueled by increasing implementation of nationwide screening initiatives, awareness campaigns about early detection, and technological advancements in imaging and diagnostic tools. Early detection of Stage III cancer enables timely surgical procedures, precise staging, and targeted therapeutic planning, thereby enhancing overall survival. Rising patient awareness, government-supported cancer screening programs, and improved access to specialized diagnostic facilities in both developed and emerging markets are driving the rapid growth of this segment in the global colorectal cancer diagnostics market.
• By Cancer Type
On the basis of cancer type, the colorectal cancer diagnostics market is segmented into adenocarcinoma, colorectal lymphoma, gastrointestinal stromal tumors, carcinoid tumors, and others. Adenocarcinoma dominated the market with a revenue share of 44.3% in 2024, underscoring its position as the most common histological subtype of colorectal cancer globally. Its market dominance is driven by its high prevalence, well-established clinical guidelines for detection, and widespread inclusion in routine colorectal cancer screening programs. The segment benefits from extensive research, standardized treatment protocols, and a strong emphasis on early detection, which collectively ensure a steady demand for diagnostic solutions targeting adenocarcinoma.
Gastrointestinal stromal tumors are projected to witness the fastest CAGR of 12.5% from 2025 to 2032, fueled by increasing awareness of rare tumor types, advancements in diagnostic imaging and molecular testing, and rising investment in specialized research programs. The segment’s growth is further supported by the development of targeted therapies and precision medicine approaches, which enable accurate detection, improved patient outcomes, and effective disease management. As healthcare providers focus on early identification and personalized treatment of rare cancers, the demand for advanced diagnostic tools in this segment is expected to rise significantly.
• By Age Group
On the basis of age group, the colorectal cancer diagnostics market is segmented into geriatric, adults, and pediatric. Adults held the largest market revenue share of 49.1% in 2024, reflecting the higher incidence of colorectal cancer among individuals aged 45–65 and their active participation in regular screening and preventive healthcare programs. This segment benefits from extensive awareness campaigns, routine check-ups, and the availability of advanced diagnostic technologies that encourage timely detection.
The geriatric segment is projected to witness the fastest CAGR of 11.9% from 2025 to 2032, driven by the increasing aging population worldwide, who are more susceptible to colorectal cancer due to age-related risk factors. Growth in this segment is further accelerated by the emphasis on preventive healthcare for older adults, widespread implementation of age-specific screening initiatives, and improved access to diagnostic services in both urban and semi-urban regions. The rising focus on early detection among geriatric patients is critical for reducing mortality rates and enhancing the overall quality of life, highlighting the segment’s growing importance in the global colorectal cancer diagnostics market.
• By End-User
On the basis of end-user, the colorectal cancer diagnostics market is segmented into hospitals, diagnostic centers, cancer research centers, ambulatory surgical centers, academic institutes, and others. Hospitals dominated the market with a revenue share of 51.4% in 2024, driven by their well-established diagnostic infrastructure, access to advanced imaging and laboratory technologies, and the presence of skilled oncology specialists. Hospitals are capable of providing end-to-end patient care, including initial screening, confirmatory diagnostics, treatment planning, therapy administration, and ongoing follow-up, making them a central hub for colorectal cancer management.
The diagnostic centers segment is expected to witness the fastest CAGR of 13.4% from 2025 to 2032, propelled by the growing patient preference for accessible, outpatient-based diagnostic services. The expansion of preventive healthcare initiatives, increasing awareness about the importance of early detection, and the proliferation of screening programs in both urban and semi-urban areas are enhancing the reach of diagnostic centers. These centers offer timely, convenient, and specialized colorectal cancer testing services, often with shorter wait times and flexible appointment options, making them increasingly popular among patients seeking prompt and efficient diagnostics.
Colorectal Cancer Diagnostics Market Regional Analysis
- North America dominated the colorectal cancer diagnostics market with the largest revenue share of 42.5% in 2024, attributed to a well-established healthcare infrastructure, high adoption of advanced screening technologies, and significant investments by key market players
- The market has experienced substantial growth in colorectal cancer diagnostic implementations across hospitals, diagnostic imaging centers, and multi-specialty clinics, driven by innovations such as AI-assisted imaging, liquid biopsy technologies, and early detection programs
- High healthcare expenditure and increasing awareness about preventive care further contribute to market expansion in the region
U.S. Colorectal Cancer Diagnostics Market Insight
The U.S. colorectal cancer diagnostics market captured the largest revenue share of 45% in North America in 2024, driven by a well-established healthcare infrastructure, high adoption of advanced screening technologies, and substantial investments by key market players. The country has witnessed rapid implementation of colorectal cancer diagnostics across hospitals, diagnostic imaging centers, and multi-specialty clinics, supported by AI-assisted imaging, liquid biopsy innovations, and early detection programs. In addition, rising demand for minimally invasive tests, integration of molecular diagnostics, and the expansion of outpatient and specialty care centers are bolstering market growth
Europe Colorectal Cancer Diagnostics Market Insight
The Europe colorectal cancer diagnostics market is projected to expand at a notable CAGR during the forecast period, supported by robust healthcare systems, increasing awareness about early cancer detection, and government initiatives promoting screening programs. Countries such as Germany, the U.K., and France are witnessing growing adoption of minimally invasive diagnostics and molecular testing solutions. The expansion of outpatient diagnostic centers and collaborations between healthcare providers and diagnostic companies are also fueling market growth across residential and clinical settings.
U.K. Colorectal Cancer Diagnostics Market Insight
The U.K. colorectal cancer diagnostics market is anticipated to grow at a significant CAGR, driven by national screening initiatives, rising incidence of colorectal cancer, and increasing preference for early detection. The country’s strong healthcare infrastructure, combined with growing investments in diagnostic technologies and integration of advanced imaging techniques, is fostering the adoption of innovative colorectal cancer testing solutions across hospitals, diagnostic centers, and research institutions.
Germany Colorectal Cancer Diagnostics Market Insight
Germany’s colorectal cancer diagnostics market is expected to expand steadily due to high healthcare expenditure, growing prevalence of colorectal cancer, and rising adoption of precision diagnostics. Increasing awareness about non-invasive testing methods, along with investments in AI-based imaging and molecular diagnostic platforms, is promoting growth in both hospital-based and standalone diagnostic facilities.
Asia-Pacific Colorectal Cancer Diagnostics Market Insight
The Asia-Pacific colorectal cancer diagnostics market is poised to grow at the fastest CAGR of 7.8% during the forecast period, driven by increasing urbanization, rising disposable incomes, expanding healthcare infrastructure, and growing awareness about cancer screening programs. Countries such as China, India, and Japan are witnessing rapid adoption of advanced diagnostics, including molecular testing, liquid biopsies, and imaging solutions, to meet the rising demand for early detection and personalized treatment strategies. Government initiatives supporting cancer screening programs and healthcare modernization are further accelerating market expansion.
Japan Colorectal Cancer Diagnostics Market Insight
The Japan colorectal cancer diagnostics market is gaining momentum due to the country’s aging population, strong healthcare system, and increasing focus on preventive oncology. Rising adoption of high-precision imaging, endoscopic technologies, and molecular diagnostic platforms is driving growth in hospitals, specialty clinics, and research centers. National screening programs and increasing patient awareness are supporting sustained market development.
China Colorectal Cancer Diagnostics Market Insight
China colorectal cancer diagnostics market accounted for the largest market revenue share within Asia-Pacific in 2024, attributed to rapid urbanization, expanding healthcare infrastructure, rising middle-class population, and growing awareness about early cancer detection. The country is experiencing increasing adoption of advanced diagnostics, such as non-invasive stool tests, imaging modalities, and liquid biopsies. Strong government support, coupled with the presence of domestic manufacturers offering cost-effective solutions, is further propelling market growth in hospitals, diagnostic centers, and specialty clinics.
Colorectal Cancer Diagnostics Market Share
The colorectal cancer diagnostics industry is primarily led by well-established companies, including:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Abbott (U.S.)
- Illumina (U.S.)
- QIAGEN (Netherlands)
- Thermo Fisher Scientific Inc. (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Merck KGaA (Germany)
- Hologic, Inc. (U.S.)
- Siemens Healthineers AG (Germany)
- BD (U.S.)
- Myriad Genetics, Inc. (U.S.)
- PlexBio Co., Ltd. (South Korea)
- FUJIFILM Corporation (Japan)
- CANON MEDICAL SYSTEMS CORPORATION (Japan)
- Medonica Co. LTD (South Korea)
- MinFound Medical Systems Co., Ltd (China)
- Bio-Rad Laboratories, Inc. (U.S.)
- Neusoft Corporation (China)
- BioFire Diagnostics (U.S.)
- Agilent Technologies, Inc. (U.S.)
Latest Developments in Global Colorectal Cancer Diagnostics Market
- In July 2024, Guardant Health announced that its Shield blood test received FDA approval as a primary screening option for colorectal cancer in adults aged 45 and older at average risk. This approval was based on the results of the ECLIPSE study, a 20,000+ patient registrational study evaluating the test's performance. The Shield test detects DNA mutations and epigenomic alterations associated with colorectal cancer, offering a non-invasive alternative to traditional screening methods
- In April 2024, Freenome announced the topline results of its PREEMPT CRC study, validating the first version of its blood-based test for early colorectal cancer detection. The study met all primary endpoints, including 79.2% sensitivity for colorectal cancer and 91.5% specificity for non-advanced colorectal neoplasia. PREEMPT CRC is the largest study of a blood-based test for colorectal cancer, enrolling 48,995 participants, reflecting the real-world diversity of average-risk adults in the U.S.
- In October 2023, the National Cancer Institute published findings on the integration of computer-aided detection (CAD) in colonoscopy procedures. The study highlighted that CAD systems could assist clinicians in identifying more polyps during colonoscopies, potentially improving the detection rates of colorectal cancer. However, the research also noted that CAD systems might not always differentiate between polyps that are likely to progress to cancer and those that are not
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.