Die steigende Nachfrage nach Pyrogenprodukten sowie Partnerschaften dürften das Marktwachstum vorantreiben. Fujifilm Wako Chemicals bietet beispielsweise Endotoxin-Testgeräte wie das Toxinometer ET-6000 an. Dieses Gerät ermöglicht drei Testarten: Gel-Clot, KTA (kinetische turbidimetrische Analyse) und KCA (kinetische chromogene Analyse). Das Toxinometer vereinfacht die Testdurchführung, da es einen neuen Test starten kann, während ein anderer noch läuft. Dies erleichtert die erneute Prüfung einer Probe. Darüber hinaus plant Fujifilm Wako Chemicals die Einführung der Toxinometer ET-7000-Serie im Jahr 2019, eines computergesteuerten Geräts mit verbesserter Benutzerfreundlichkeit. Infolgedessen führen vermehrte Produkteinführungen letztendlich zu Marktwachstum.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/global-endotoxin-and-pyrogen-testing-market
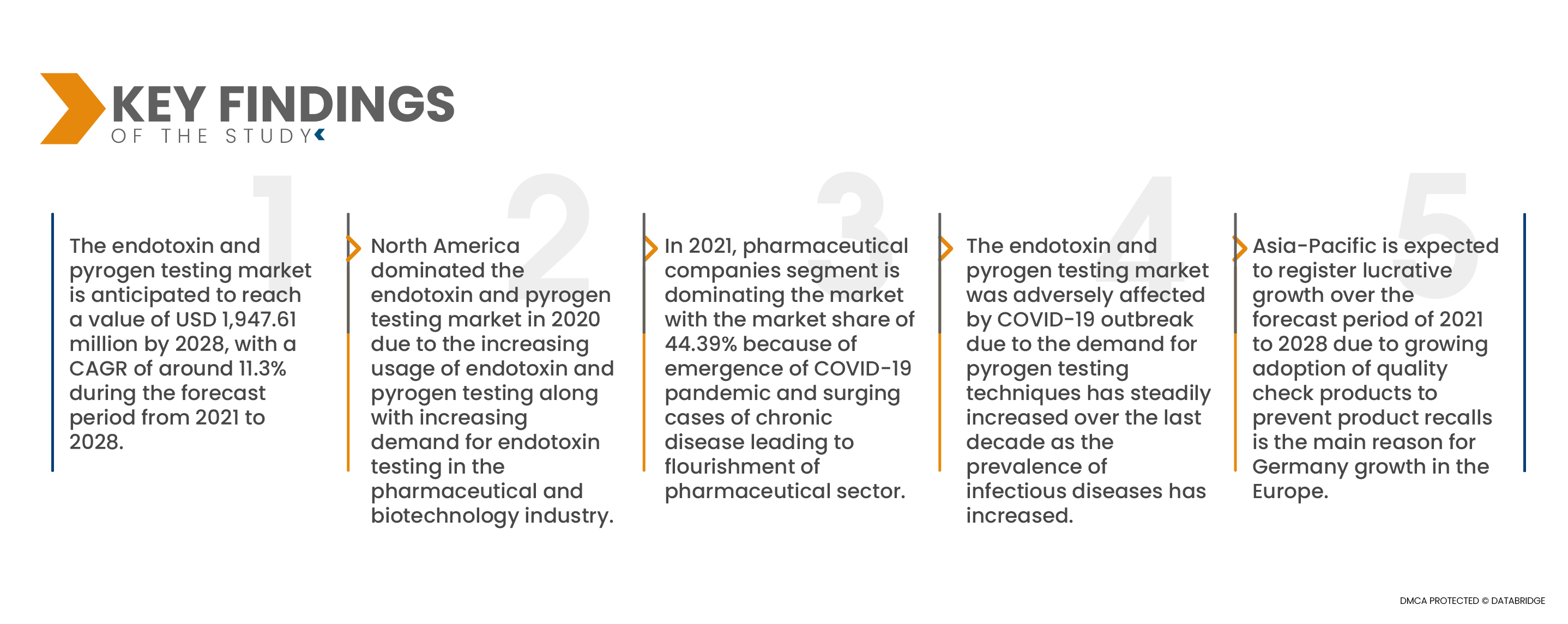
Data Bridge Market Research analysiert, dass der Markt für Endotoxin- und Pyrogentests im Prognosezeitraum von 2021 bis 2028 voraussichtlich mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 11,3 % wachsen und bis 2028 voraussichtlich 1.947,61 Millionen US-Dollar erreichen wird.
Es wird erwartet, dass die steigenden Ausgaben für Forschung und Entwicklung den Markt ankurbeln
Steigende Ausgaben für Forschung und Entwicklung trugen ebenfalls zum allgemeinen Marktwachstum bei. Laut dem Bericht der European Federation of Pharmaceutical Industries and Associations (EFPIA) aus dem Jahr 2019 investierte die europäische Pharmaindustrie im Jahr 2018 rund 36,5 Milliarden Euro in Forschung und Entwicklung. Die schnell wachsende Pharma- und Biotechnologiebranche dürfte das Marktwachstum weiter vorantreiben. Pharma-, Biotech- und andere Arzneimittelhersteller nutzen Pyrogentests, um die sichere Herstellung und Markteinführung infektionsfreier Produkte zu gewährleisten.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2020 bis 2027
|
Basisjahr
|
2019
|
Historische Jahre
|
2018 (Anpassbar auf 2012 – 2017)
|
Quantitative Einheiten
|
Umsatz in Millionen USD, Mengen in Einheiten, Preise in USD
|
Abgedeckte Segmente
|
Typ (vorgefertigte Endotoxin- und Pyrogentests, Proendotoxin- und Pyrogentests und kombinierte Endotoxin- und Pyrogentests), Produktkategorie (Clean Labeled Ingredient und konventionell), Form (Pulver und Flüssigkeit), Anwendung ( Pharmazeutika , Lebensmittel, Tierfutter, Körperpflege und Kosmetik, Nahrungsergänzungsmittel, Getränke und andere)
|
Abgedeckte Länder
|
USA, Kanada, Mexiko in Nordamerika, Deutschland, Schweden, Polen, Dänemark, Italien, Großbritannien, Frankreich, Spanien, Niederlande, Belgien, Schweiz, Türkei, Russland, Restliches Europa in Europa, Japan, China, Indien, Südkorea, Neuseeland, Vietnam, Australien, Singapur, Malaysia, Thailand, Indonesien, Philippinen, Restlicher Asien-Pazifik-Raum (APAC) in Asien-Pazifik (APAC), Brasilien, Argentinien, Restliches Südamerika als Teil von Südamerika, VAE, Saudi-Arabien, Oman, Katar, Kuwait, Südafrika, Restlicher Naher Osten und Afrika (MEA) als Teil von Naher Osten und Afrika (MEA)
|
Abgedeckte Marktteilnehmer
|
Eurofins Scientific (Luxemburg), Thermo Fisher Scientific Inc. (USA), Pall Corporation (eine Tochtergesellschaft von Danaher) (USA), Lonza (Schweiz), Charles River Laboratories (USA), Merck KGaA (Deutschland), STERIS plc (USA), SGS SA (Schweiz), Sartorius AG (Deutschland), bioMérieux SA (Frankreich), Ellab A/S (Dänemark), Wako USA (eine Tochtergesellschaft von FUJIFILM Wako Pure Chemical Corporation) (USA), ASSOCIATES OF CAPE COD, INC. (USA), WuXi AppTec (China), Microcoat Biotechnologie GmbH (Deutschland) und andere.
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, geografisch dargestellte Produktion und Kapazität nach Unternehmen, Netzwerklayouts von Distributoren und Partnern, detaillierte und aktuelle Preistrendanalysen und Defizitanalysen der Lieferkette und Nachfrage.
|
Segmentanalyse:
Der globale Markt für Endotoxin- und Pyrogentests ist in sieben wichtige Segmente unterteilt: Produkttyp, Testtyp, Anwendung, Methode, Kaufart, Endprodukt und Endbenutzer.
- Auf der Grundlage des Produkttyps ist der Markt für Endotoxin- und Pyrogentests in Nachweiskits und Reagenzien, Instrumente, Systeme und Software, Endotoxintestdienste sowie Verbrauchsmaterialien und Zubehör unterteilt.
Im Jahr 2021 dominiert das Segment der Nachweiskits und Reagenzien den Markt für Endotoxin- und Pyrogentests
Das Segment der Detektionskits und Reagenzien dominiert den Markt mit einem Marktanteil von 59,73 % aufgrund des technologischen Fortschritts bei der Entwicklung effizienter Kits und Reagenzien für die Qualitätskontrolle im Gesundheitssektor.
- Auf der Grundlage des Testtyps ist der Markt für Endotoxin- und Pyrogentests in Limulus-Amöbozytenlysat-Test (LAL), TAL-Test, Monozytenaktivierungstest (MAT), Kaninchen-Pyrogentest und rekombinanten C-Test (RFC) unterteilt.
Im Jahr 2021 dominiert das Limulus-Amöbozytenlysat (LAL)-Testsegment den Markt für Endotoxin- und Pyrogentests
Das Testsegment Limulus-Amöbozytenlysat (LAL) dominiert den Markt mit einem Marktanteil von 56,50 % aufgrund der gestiegenen Effizienz und Zuverlässigkeit verschiedener Verbraucher- und Aufsichtsbehörden hinsichtlich dieses speziellen Testtyps für Endotoxintests.
- Der Markt für Endotoxin- und Pyrogentests ist nach Anwendungsgebieten in die Bereiche Arzneimittelherstellung, Medizinprodukteherstellung , Rohstoffproduktion und Verpackungsherstellung unterteilt. Im Jahr 2021 dominiert die Arzneimittelherstellung den Markt mit einem Marktanteil von 34,86 %, da der florierende Pharmasektor die Nachfrage nach zunehmenden chronischen Krankheiten weltweit deckt.
- Der Markt für Endotoxin- und Pyrogentests ist methodisch in Gel-Clot-Endotoxin-Tests, chromogene Endotoxin-Tests und turbidimetrische Endotoxin-Tests unterteilt. Im Jahr 2021 dominiert das Segment der Gel-Clot-Endotoxin-Tests den Markt mit einem Marktanteil von 55,45 %, da Modifikationen der Gel-Clot-Methode den Bedarf an LAL-Reagenzien im Vergleich zur Basismethode verringern.
- Der Markt für Endotoxin- und Pyrogentests ist nach Kaufart in Großgruppen, mittlere und kleine Gruppen sowie Einzelpersonen segmentiert. Im Jahr 2021 dominiert das Großgruppensegment den Markt mit einem Marktanteil von 60,07 %, da Großgruppen für jedes wachsende Unternehmen oder jede wachsende Firma im Markt für Endotoxin- und Pyrogentests eine wirtschaftliche Kaufoption darstellen.
- Der Markt für Endotoxin- und Pyrogentests ist nach Endprodukt in Impfstoffe und/oder CGT, Biologika, Injektionsmittel und weitere Produkte segmentiert. Im Jahr 2021 dominiert das Segment Impfstoffe und/oder CGT den Markt mit einem Marktanteil von 59,76 %, da die Forschung und Entwicklung für die Entwicklung von Impfstoffen gegen COVID-19 weltweit zunimmt.
- Der Markt für Endotoxin- und Pyrogentests ist nach Endverbrauchern in Pharmaunternehmen, Biotechnologieunternehmen, Biomedizinunternehmen, Medizinproduktehersteller, Auftragsforschungsinstitute (CROs) und Auftragshersteller (CMOs) segmentiert. Im Jahr 2021 dominiert das Segment der Pharmaunternehmen mit einem Marktanteil von 44,39 % den Markt. Grund hierfür sind das Auftreten der COVID-19-Pandemie und die steigende Zahl chronischer Erkrankungen, die zu einem Aufschwung der Pharmabranche führten.
Hauptakteure
Data Bridge Market Research erkennt die folgenden Unternehmen als die wichtigsten Akteure auf dem Markt für Endotoxin- und Pyrogentests an: Eurofins Scientific (Luxemburg), Thermo Fisher Scientific Inc. (USA), Pall Corporation (eine Tochtergesellschaft von Danaher) (USA), Lonza (Schweiz), Charles River Laboratories (USA), Merck KGaA (Deutschland), STERIS plc (USA), SGS SA (Schweiz), Sartorius AG (Deutschland), bioMérieux SA (Frankreich), Ellab A/S (Dänemark), Wako USA (eine Tochtergesellschaft von FUJIFILM Wako Pure Chemical Corporation) (USA), ASSOCIATES OF CAPE COD, INC. (USA), WuXi AppTec (China), Microcoat Biotechnologie GmbH (Deutschland) und andere.
Marktentwicklung
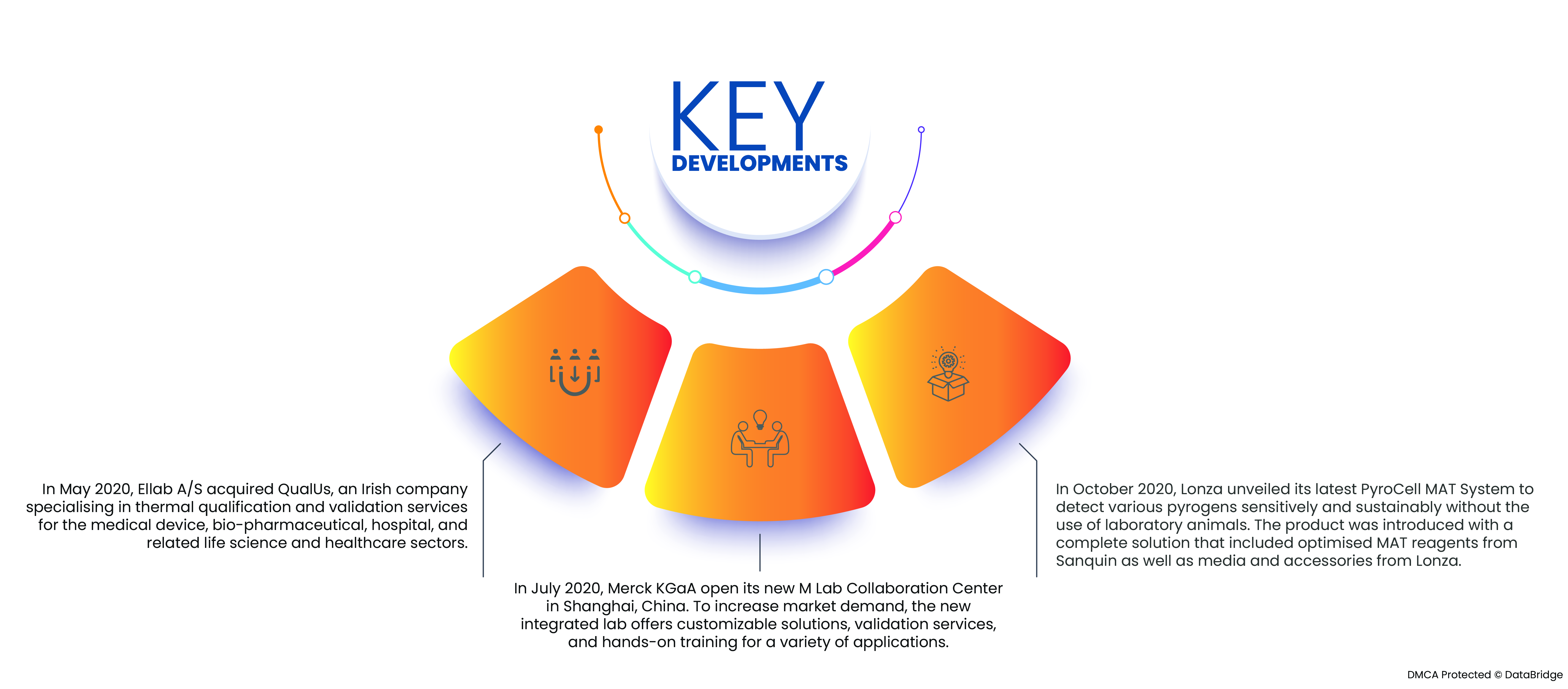
- Im Mai 2020 übernahm Ellab A/S QualUs, ein irisches Unternehmen, das sich auf thermische Qualifizierungs- und Validierungsdienstleistungen für die Bereiche Medizintechnik, Biopharmazie, Krankenhäuser sowie verwandte Biowissenschaften und Gesundheitswesen spezialisiert hat. Die Akquisition stärkt das Produktportfolio des Unternehmens und führt zukünftig zu einer höheren Nachfrage und höheren Umsätzen.
- Im Juli 2020 eröffnete die Merck KGaA ihr neues M Lab Collaboration Center in Shanghai, China. Um die Marktnachfrage zu steigern, bietet das neue integrierte Labor maßgeschneiderte Lösungen, Validierungsservices und praxisorientierte Schulungen für verschiedene Anwendungen. Das neue Produktionslabor des Unternehmens wird die Produktionskapazität erhöhen und so zukünftig die Produktverfügbarkeit und den Umsatz steigern.
- Im Oktober 2020 stellte Lonza sein neuestes PyroCell MAT-System vor, das verschiedene Pyrogene empfindlich und nachhaltig ohne den Einsatz von Labortieren nachweist. Das Produkt wurde mit einer Komplettlösung eingeführt, die optimierte MAT-Reagenzien von Sanquin sowie Medien und Zubehör von Lonza umfasste. Die Einführung dieses neuen Produkts hat die Glaubwürdigkeit des Unternehmens und die Marktnachfrage erhöht.
Regionale Analyse
Geografisch betrachtet sind die im Marktbericht für Endotoxin- und Pyrogentests abgedeckten Länder die USA, Kanada, Mexiko in Nordamerika, Deutschland, Schweden, Polen, Dänemark, Italien, Großbritannien, Frankreich, Spanien, die Niederlande, Belgien, die Schweiz, die Türkei, Russland, das übrige Europa in Europa, Japan, China, Indien, Südkorea, Neuseeland, Vietnam, Australien, Singapur, Malaysia, Thailand, Indonesien, die Philippinen, der übrige asiatisch-pazifische Raum (APAC) in Asien-Pazifik (APAC), Brasilien, Argentinien, der übrige Südamerika als Teil von Südamerika, die Vereinigten Arabischen Emirate, Saudi-Arabien, Oman, Katar, Kuwait, Südafrika, der übrige Nahe Osten und Afrika (MEA) als Teil von Naher Osten und Afrika (MEA).
Laut Marktforschungsanalyse von Data Bridge:
Nordamerika ist die dominierende Region im Markt für Endotoxin- und Pyrogentests im Prognosezeitraum 2021 – 2028
Im Jahr 2020 dominierte Nordamerika den Markt für Endotoxin- und Pyrogentests aufgrund eines großen Anteils am US-Markt aufgrund der zunehmenden Verwendung von Endotoxin- und Pyrogentests sowie der steigenden Nachfrage nach Endotoxintests in der Pharma- und Biotechnologiebranche.
Der asiatisch-pazifische Raum wird voraussichtlich im Prognosezeitraum 2021 – 2028 die am schnellsten wachsende Region im Markt für Endotoxin- und Pyrogentests sein.
Im asiatisch-pazifischen Raum wird im Prognosezeitraum ein Wachstum erwartet, da die zunehmende Einführung von Qualitätskontrollprodukten zur Vermeidung von Produktrückrufen der Hauptgrund für das Wachstum Deutschlands in Europa ist.
COVID-19-Auswirkungsanalyse
Mit der COVID-19-Pandemie ist die Nachfrage nach Pyrogentests im letzten Jahrzehnt stetig gestiegen, da die Prävalenz von Infektionskrankheiten zunimmt. Es wird erwartet, dass die Nachfrage nach Pyrogentests im Prognosezeitraum aufgrund der zunehmenden Inzidenz von Infektionskrankheiten weiter steigen wird. Produkte zur Endotoxin-Identifizierung können dazu beitragen, das Management von Infektionskrankheiten zu verbessern, insbesondere in Gebieten mit eingeschränkter Gesundheitsinfrastruktur. Dies hat zu einer zunehmenden Nutzung von Pyrogentests geführt und unterstützt das Marktwachstum.
Für detailliertere Informationen zum Marktbericht für Endotoxin- und Pyrogentests klicken Sie hier – https://www.databridgemarketresearch.com/reports/global-endotoxin-and-pyrogen-testing-market










