A hernia is a disease characterized by the protrusion of an organ or tissue through a weakened or damaged area of the body wall. It usually occurs in the abdomen but can also occur in other places, such as the groin, diaphragm, or navel (belly).
The increase in the incidence of hernias can be due to several factors. One of the most important factors is the aging of the population. As people age, the natural aging process affects the body's connective tissues, including those that support the abdominal wall. This gradual weakening of the connective tissues makes them more susceptible to herniation.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-hernia-repair-devices-market
Thus, the increasing incidence of hernia is expected to act as a driver for market growth.
Data Bridge Market Research analyzes that the Global (U.S., Canada, U.K., Germany, Italy, France, Spain, Japan, India, China, Australia) Hernia Repair Devices (Permanent and Absorbable Hernia Fixation) Market is expected to grow with a CAGR of 13.5% in the forecast period of 2023 to 2030 and is expected to reach USD 6,717.76 million by 2030. The hernia mesh segment is projected to propel the market growth as an increasing demand for bioresorbable hernia fixation devices.
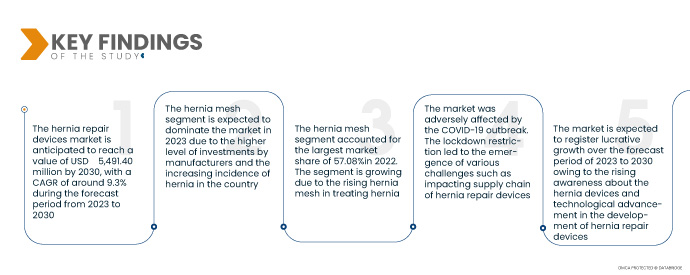
Key Findings of the Study
Growing demand for minimally invasive surgeries
Minimally invasive surgery is associated with less pain, a shorter hospital stay, and fewer complications, increasing its demand in the market. Hernia minimal invasive surgeries are growing in the market with the increase in demand for minimally invasive techniques and their benefits to the patients compared to traditional open surgeries, which pose risks of wound infection among others.
In recent years, due to several factors, the demand for minimally invasive surgeries related to hernia treatment has increased. Minimally invasive surgeries, also called laparoscopic or keyhole surgeries, involve small incisions and special tools, and a camera to perform the procedure. This approach has several advantages over traditional open surgery for hernia treatment.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD million
|
|
Segments Covered
|
Product (Hernia Mesh, Hernia Fixation Devices, and Others), Surgery (Open Surgery, Laparoscopic Surgery, and Robotic Surgery), Type (Inguinal Hernia, Ventral Hernia, Femoral Hernia, Hiatal (Hiatus) Hernia, and Diaphragmatic Hernia), Device Type (Permanent and Absorbable Hernia Fixation), End User (Hospitals, Specialty Clinic, Ambulatory Surgical Centers, and Others)
|
|
Countries Covered
|
U.S., Canada, U.K., Germany, Italy, France, Spain, Japan, India, China, and Australia
|
|
Market Players Covered
|
Medtronic (Ireland), BD (U.S.), Cook Group (U.S.), Inc., W. L. Gore & Associates, Inc. (U.S.), ETHICON (A subsidiary of Johnson & Johnson Services, Inc.) (U.S.), Vivostat A/S (Denmark), Baxter (U.S.), Cooper Surgical Inc. (A Division of The Cooper Companies Inc.) (U.S.), BioCer Development GmbH (Germany), pfm medical ag 9Germany), B. Braun SE (Germany), Integra LifeSciences (U.S.), AbbVie Inc. (U.S.), BIOSISHEALING (China), Dipromed Srl (Italy), DemeTECH (U.S.), and Cousin Surgery (France) among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research include depth expert analysis, premium insights.
|
Segment Analysis:
The market is segmented into six notable segments based on product, surgery type, type, device type, end user, and distribution channel.
- On the basis of product, the market is segmented into hernia mesh, hernia fixation devices, and others.
In 2023, hernia mesh is dominating the hernia repair devices (permanent and absorbable hernia fixation) market.
In 2023, hernia mesh is dominating the hernia repair devices (permanent and absorbable hernia fixation) market owing to the increasing demand for hernia mesh in hernia repair surgeries due to its effectiveness in providing support and reinforcing the weakened or damaged tissue.
- On the basis of surgery type, the market is segmented into open surgery, laparoscopic surgery, and robotic surgery. In 2023, the open surgery segment is expected to dominate the market with 45.46% market share and is expected to reach USD 2,573.67 million by 2030 from USD 1,254.57 million in 2022, growing with the highest CAGR of 9.7% in the forecast period of 2023 to 2030
- On the basis of type, the market is segmented into inguinal hernia, ventral hernia, hiatal (hiatus) hernia, femoral hernia, and diaphragmatic hernia.
In 2023, the inguinal hernia segment is expected to dominate the market
In 2023, the inguinal hernia segment is expected to dominate the market with 48.66% market share and is expected to reach USD 2,793.99 million by 2030 from USD 1,339.90 million in 2022, growing with the highest CAGR of 10.0% in the forecast period of 2023 to 2030
- On the basis of device type, the market is segmented into permanent and absorbable hernia fixation. In 2023, the permanent segment is expected to dominate the market with 72.35% market share and is expected to reach USD 4,126.10 million by 2030 from USD 1,993.69 million in 2022, growing with the highest CAGR of 9.9% in the forecast period of 2023 to 2030
- On the basis of end user, the market is segmented into hospitals, specialty centers, ambulatory centers, and others. In 2022, the hospitals segment is expected to dominate the market with 59.23% market share and is expected to reach USD 3,463.00 million by 2030 from USD 1,625.77 million in 2022, growing with the highest CAGR of 10.3% in the forecast period of 2023 to 2030
- On the basis of distribution type, the market is segmented into retail sales and direct tender. In 2022, the retail sales segment is expected to dominate the market with 70.11% market share and is expected to reach USD 4,012.28 million by 2030 from USD 1,931.06 million in 2022, growing with the highest CAGR of 9.9% in the forecast period of 2023 to 2030.
Major Players
Data Bridge Market Research recognizes the following companies as the market players in the market are Medtronic (Ireland), BD (U.S.), Cook Group (U.S.), Inc., W. L. Gore & Associates, Inc. (U.S.), ETHICON (A subsidiary of Johnson & Johnson Services, Inc.) (U.S.), Vivostat A/S (Denmark), Baxter (U.S.), Cooper Surgical Inc. (A Division of The Cooper Companies Inc.) (U.S.), BioCer Development GmbH (Germany), pfm medical ag 9Germany), B. Braun SE (Germany), Integra LifeSciences (U.S.), AbbVie Inc. (U.S.), BIOSISHEALING (China), Dipromed Srl (Italy), DemeTECH (U.S.), and Cousin Surgery (France) among others.
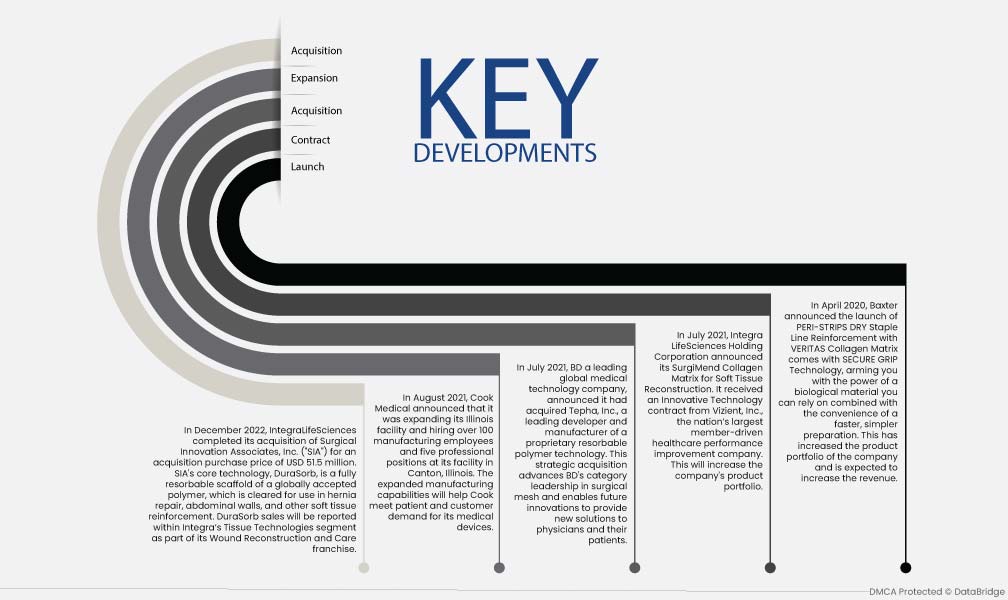
Market Development
- In December 2022, IntegraLifeSciences completed its acquisition of Surgical Innovation Associates, Inc. ("SIA") for an acquisition purchase price of USD 51.5 million. SIA's core technology, DuraSorb, is a fully resorbable scaffold of a globally accepted polymer, which is cleared for use in hernia repair, abdominal walls, and other soft tissue reinforcement. DuraSorb sales will be reported within Integra’s Tissue Technologies segment as part of its Wound Reconstruction and Care franchise
- In August 2021, Cook Medical announced that it was expanding its Illinois facility and hiring over 100 manufacturing employees and five professional positions at its facility in Canton, Illinois. The expanded manufacturing capabilities will help Cook meet patient and customer demand for its medical devices
- In July 2021, BD, a leading global medical technology company, announced it had acquired Tepha, Inc., a leading developer and manufacturer of proprietary resorbable polymer technology. This strategic acquisition advances BD's category leadership in surgical mesh and enables future innovations to provide new solutions to physicians and their patients
- In July 2021, Integra LifeSciences Holding Corporation announced its SurgiMend Collagen Matrix for Soft Tissue Reconstruction. It received an Innovative Technology contract from Vizient, Inc., the nation’s largest member-driven healthcare performance improvement company. This will increase the company's product portfolio
- In April 2020, Baxter announced the launch of PERI-STRIPS DRY Staple Line Reinforcement with VERITAS Collagen Matrix comes with SECURE GRIP Technology, arming you with the power of a biological material you can rely on combined with the convenience of a faster, simpler preparation. This has increased the product portfolio of the company and is expected to increase the revenue
For more detailed information about the medical foods for inborn errors of metabolism market report, click here – https://www.databridgemarketresearch.com/reports/global-hernia-repair-devices-market