The number of people suffering from kidney disorders is rising, which surges the burden of this disease in the healthcare system. The treatment process is very expensive. It becomes unaffordable for many of the people as the novel drugs are costly. However, the U.S. and other developed nations having larger market share and have a well-managed reimbursement policy for kidney disorders programs which are expected to enhance the market growth in the forecast period.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-iga-nephropathy-market
Data Bridge Market Research analyses that the Immunoglobulin A (IgA) Nephropathy Market expected to reach USD 370.87 million by 2029 from USD 85.68 million in 2021, growing with a CAGR of 20.10% in the forecast period of 2022 to 2029. Increasing collaboration between major market players for drug development will likely to increase the growth of the global immunoglobulin A (IgA) nephropathy market.
Growing IGA targeted pipeline drugs is expected to drive the market's growth rate
The immunoglobulin A (IgA) nephropathy is a disorder that requires to be investigated systematically for getting a novel targeted treatment option. As of March 2018, the immunoglobulin A (IgA) nephropathy therapeutics pipeline includes 24 drug candidates, of which 3 drug candidates are in the 3rd stage of development. According to Clinical trials Database, in recent year nearly 128 different studies are on-going on immunoglobulin A (IgA) nephropathy. For instance, Omeros Corporation has announced that they had completed full plan for a clinical trial for OMS721 in 2019 for the treatment of immunoglobulin A (IgA) nephropathy. This illustrations that the number of pipeline drugs for targeted therapy of immunoglobulin A (IgA) nephropathy is supposed to grow in the forecast period
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014- 2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Treatment (Medication, Kidney Transplantation, Others), Diagnosis (Iothalamate Clearance Test, Kidney Biopsy, Blood Tests, Urine Tests), Disease Type (Primary IgA Nephropathy, Secondary IgA Nephropathy), Symptoms (Hematuria, Proteinuria, Edema, Others), Population Type (Pediatrics, Adults), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Market Players Covered
|
AstraZeneca (U.K.), Johnson & Johnson Private Limited (U.S.), Cipla Inc. (India), Siemens Healthcare GmbH (Germany), Zydus Cadila (India), Hikma Pharmaceuticals PLC (U.K.), LEO Pharma A/S (Denmark), Fresenius Kabi AG (Germany), Accord Healthcare (U.K.), Abbott (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Sanofi (France), Novartis AG (Switzerland), Sun Pharmaceutical Industries Ltd. (India), Aurobindo Pharma (India), Lupin (India), Alembic Pharmaceuticals Limited (India), Apotex Inc. (Canada)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The immunoglobulin A (IgA) nephropathy market is segmented on the basis of treatment, diagnosis, disease type, symptoms, population type, route of administration, end-users and distribution channel.
- On the basis of disease type, global immunoglobulin A (IgA) nephropathy market is segmented into primary IgA nephropathy, secondary IgA nephropathy. Primary immunoglobulin A (IgA) nephropathy segment is expected to dominate the market with 60.52% market share because the incidence of primary immunoglobulin A (IgA) nephropathy is higher than other and can be diagnosed by urine or blood test easily.
- On the basis of symptoms, global immunoglobulin A (IgA) nephropathy market is segmented into hematuria, proteinuria, edema and others. Hematuria segment is expected to dominate the market with 65.76% market share because it is the most commonly used parameter for clinical examination and it is easily detected by the blood in the urine by showing the occurrence of disease in the kidney.
- On the basis of type, global immunoglobulin A (IgA) nephropathy market is segmented into diagnosis and treatment.
The diagnosis segment of type segment is anticipated to dominate the immunoglobulin A (IgA) nephropathy market
Diagnosis segment is expected to dominate the market with 71.30% market share because it helps in proper detection and tracing of the immunoglobulin A (IgA) nephropathy. The tracing of the immunoglobulin A (IgA) nephropathy is possible only through the primary detection such as urine and blood test and confirmatory detection which are the major factors for the growth of this segment.
- On the basis of population type, global immunoglobulin A (IgA) nephropathy market is segmented into pediatrics and adults.
The adults segment of population type segment is anticipated to dominate the immunoglobulin A (IgA) nephropathy market
Adults segment is expected to dominate the market with 65.36% market share because with ageing the quantity of protein build in the kidney started at a certain concentration with time. This is a common problem in younger and elderly adults as a result of this adults segment is dominating the market
- On the basis of route of administration, global immunoglobulin A (IgA) nephropathy market is segmented into oral, parenteral and others. Oral segment is expected to dominate the market with 75.54% market share owing to the extensive variety of oral product portfolio present in the interstate commerce by the market which rise the market of this segment.
- On the basis of end user, the global immunoglobulin A (IgA) nephropathy market is segmented into hospitals, clinics, home healthcare and others. Hospitals segment is expected to dominate the market with 31.10% market share because the hospitals department have a professional team with well-trained staffs for proper diagnosis and treatment of the patients.
- On the basis of distribution channel, global immunoglobulin A (IgA) nephropathy market is segmented into direct tender hospital pharmacy, retail pharmacy, online pharmacy and others. Direct tender segment is expected to dominate the market with 65.52% market share because it accelerates the revenue growth and provides tax benefits.
Major Players
Data Bridge Market Research recognizes the following companies as the major immunoglobulin A (IgA) nephropathy market players in immunoglobulin A (IgA) nephropathy market AstraZeneca (U.K.), Johnson & Johnson Private Limited (U.S.), Cipla Inc. (India), Siemens Healthcare GmbH (Germany), Zydus Cadila (India), Hikma Pharmaceuticals PLC (U.K.), LEO Pharma A/S (Denmark), Fresenius Kabi AG (Germany), Accord Healthcare (U.K.), Abbott (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland).
Market Development
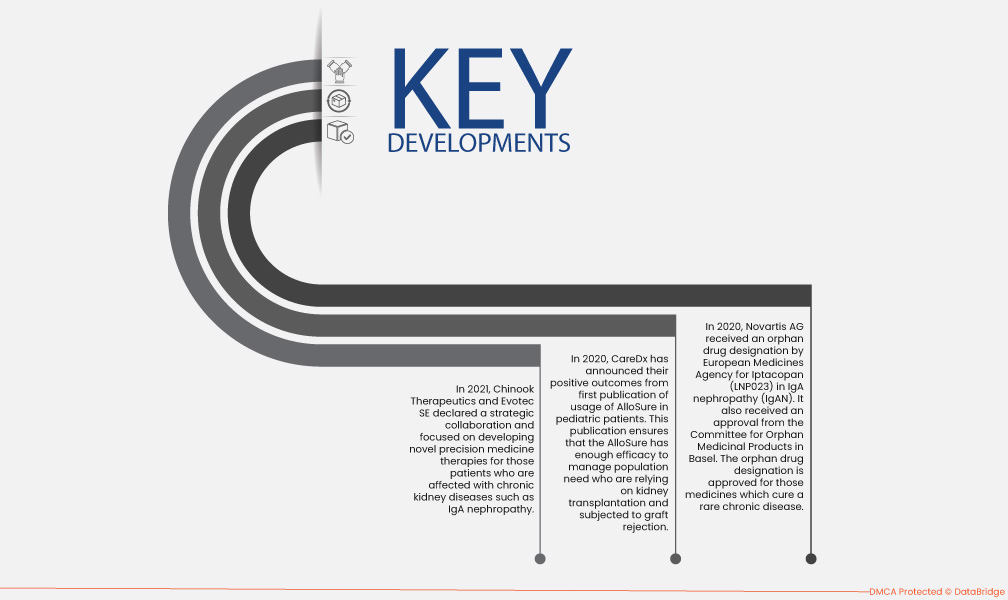
- In 2021, Chinook Therapeutics and Evotec SE declared a strategic collaboration. They focused on developing novel precision medicine therapies for patients affected with chronic kidney diseases such as IgA nephropathy. With this collaboration, enhanced throughput proteomics, high throughput transcriptomics, and cell imaging for IgA nephropathy disease
- In 2020, CareDx announced the positive outcomes from the first publication of usage of AlloSure in pediatric patients. This publication ensures that the AlloSure has enough efficacy to manage population need who are relying on kidney transplantation and subjected to graft rejection.
- In 2020, Novartis AG received an orphan drug designation by European Medicines Agency for Iptacopan (LNP023) in IgA nephropathy (IgAN). It also received an approval from the Committee for Orphan Medicinal Products in Basel. The orphan drug designation is approved for those medicines which cure a rare chronic disease. The approval received would result in rapid distribution of the drugs in kidney diagnostic centers and hospitals in Europe.
Regional Analysis
Geographically, the countries covered in the immunoglobulin A (IgA) nephropathy market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As per Data Bridge Market Research analysis:
North America is the dominant region in immunoglobulin A (IgA) nephropathy market during the forecast period 2022 to 2029
North America dominates the immunoglobulin A (IgA) nephropathy market owing to the higher level of investments by U.S. manufacturers in this region. Furthermore, presence of major key players and increasing demand for immunoglobulin A (IgA) nephropathy products will further enhance the market growth in this region.
Asia-Pacific is estimated to be the fastest-growing region in immunoglobulin A (IgA) nephropathy market the forecast period 2022 to 2029
Asia-Pacific is expected to grow during the forecast period of 2022 to 2029 owing to the presence of major market players and increasing government initiatives in this region. Additionally, the increasing development of healthcare infrastructure and growing government initiatives are some of the other major factors which will further boost the market growth in this region.
For more detailed information about immunoglobulin A (IgA) nephropathy market report, click here –https://www.databridgemarketresearch.com/reports/global-iga-nephropathy-market










