North America Iga Nephropathy Market is expected to grow with a CAGR of 19.7% in the forecast period of 2021 to 2028. The years considered for study are as mentioned below.
Access Full Report @ https://www.databridgemarketresearch.com/reports/north-america-iga-nephropathy-market
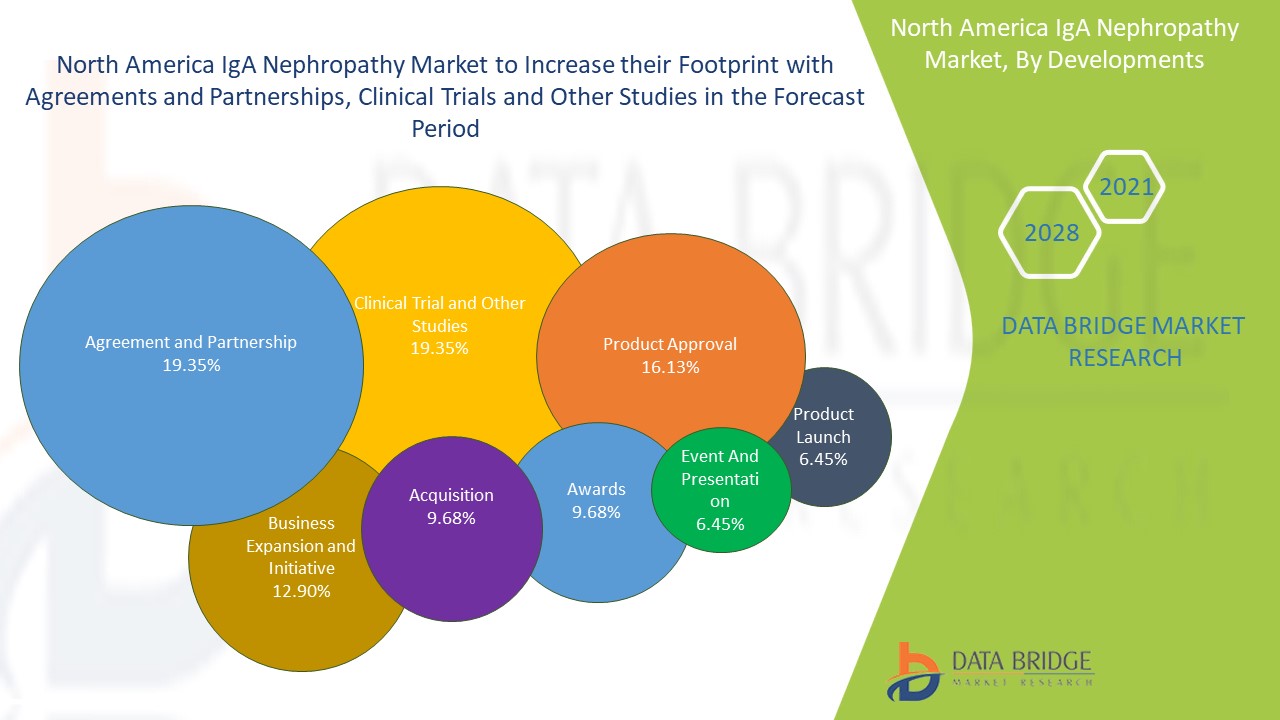
North America IgA nephropathy market is a highly expansive market, which includes a specific number of key players and local players. The market has witnessed increased pipeline drugs and clinical trials to develop a novel and innovative drug for the treatment.
The major players in the North America IgA nephropathy market are introducing a strong range of product portfolios. This helped companies to maximize sales with an enhanced product portfolio.
For instance,
- In September 2020, Viatris Inc., a combined company of Mylan and Pfizer Inc., received EU approval. Moreover, the company also achieved approval from the Australian Competition, Consumer Commission, and New Zealand Commission, strengthening its company establishment proposal. This approval allowed the company to launch a new company that is positioned to meet patients' need
Teva Pharmaceuticals USA, Inc. (a subsidiary of Teva Pharmaceutical Industries Ltd.) is the dominating player in North America IgA nephropathy market. The other key players existing in the market include Viatris Inc., AstraZeneca, Novartis AG, Pfizer Inc., Zydus Cadila, Fresenius Kabi USA (a subsidiary of Fresenius SE & Co. KGaA), Hikma Pharmaceuticals PLC, LUPIN, Accord Healthcare, Siemens Healthcare GmbH (a subsidiary of Siemens Healthineers AG), Sun Pharmaceutical Industries Ltd., ARKRAY USA, Inc., Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., CareDX, Inc., Alembic Pharmaceuticals Limited, Strides Pharma Science Limited, Apotex Inc., Caliditas Therapeutics AB, Omeros Corporation among others.
 Teva Pharmaceuticals USA, Inc.
Teva Pharmaceuticals USA, Inc.
Teva Pharmaceutical USA Inc. has its base headquarters in New Jersey, U.S. The company is focused on innovative treatments and quality generic medicines which improve health and life. The company deals in multiple product portfolios such as Product Catalogue, Generic Medicines, Specialty Medicines, Biopharmaceuticals, Report Adverse Effects, Patient Resources, Trade Partner Information, and Generic Medicines is the market-focused category.
- In October 2021, Teva Pharmaceuticals USA, Inc. had announced the launch of the FDA-approved generic versions of TRUVADAi and ATRIPLA I tablets for HIV-1 and renal failure. During the COVID-19 pandemic, access to treatment is essential than ever for those who are immunocompromised and at risk of developing more severe disease. The novel product would result in a rise in net sales and distribution of generic drugs across hospitals and diagnostic centers. It is predicted to deliver linear growth in the market
The company has a presence in the Americas, Europe, the Middle East and Africa, Asia-Pacific.
VIATRIS, INC
Viatris Inc is headquartered in Pennsylvania, U.S., and was founded in the year 1961. The company is engaged in delivering increased access to affordable, quality medicines for patients worldwide, regardless of geography or circumstances. The company offers a wide range of products in various categories such as Legacy Mylan Products, Legacy Upjohn Products, out of which Legacy Mylan Products, Legacy Upjohn Products is the market-focused category.
For instance,
- In October 2020, Viatris Inc., a combined company of Mylan and Pfizer Inc. received clearance from the U.S. Federal Trade Commission. This approval paved the way for establishing a new company, emphasizing the development of innovative medicines and breakthroughs to change patients’ lives. This approval unlocked the future for company’s growth
The company has a wide presence in Asia-Pacific, Americas, Europe. The company has its subsidiaries in Rottapharm | Madaus GmbH (Germany), MEDA Pharma GmbH, and Co. KG (Germany), Merck Generics Belgium B.V.B.A. (Belgium), Arcana Arzneimittel GmbH (Germany), Mylan Laboratories India Private Ltd (India), among others.
NOVARTIS AG
Novartis AG is headquartered in Basel, Switzerland, and was founded in 1996. The company is engaged in investing in the most promising frontiers of science, the most exciting innovation in the business of medicines, and the most significant healthcare needs. The company's various products include Cancer, Cardiovascular, Renal and Metabolism, Immunology and Dermatology, Ophthalmology, Neuroscience, and Respiratory. Out of which, Renal and Metabolism is the market-focused category.
For instance,
- In December 2020, Novartis AG had announced the U.S. FDA had approved the breakthrough therapy designation for iptacopan (LNP023), in paroxysmal nocturnal hemoglobinuria (PNH) and rare paediatric disease (RPD) Designation in C3 glomerulopathy (C3G). The breakthrough designation would process the development and review of medicines to address the chronic medical needs in kidney disorders. It would result in timely treatment of the patients and increase the demand for novel therapies in the forecast period
The company has a presence in Asia-Pacific, Europe, the Americas, Middle East, and Africa. The company has its subsidiaries in Novartis Australia Pty Ltd (Australia), Novartis Austria GmbH (Austria), Novartis Pharma NV (Belgium), Novartis Securities Investment Ltd. (Hamilton), Novartis Biociências S.A. (Brazil), Sandoz (China) Pharmaceutical (China) among others.










