The Mexico dimenhydrinate market is experiencing a surge in demand for motion sickness relief and antihistamine solutions. Dimenhydrinate, known for addressing travel-induced nausea, is gaining popularity with heightened consumer awareness. Pharmaceutical firms are responding by introducing innovative formulations to meet diverse preferences. This dynamic market signifies the convergence of healthcare needs and ongoing pharmaceutical advancements, highlighting a pivotal intersection in addressing motion-related discomfort and fostering overall wellness in Mexico.
Access Full Report @ https://www.databridgemarketresearch.com/reports/mexico-dimenhydrinate-market
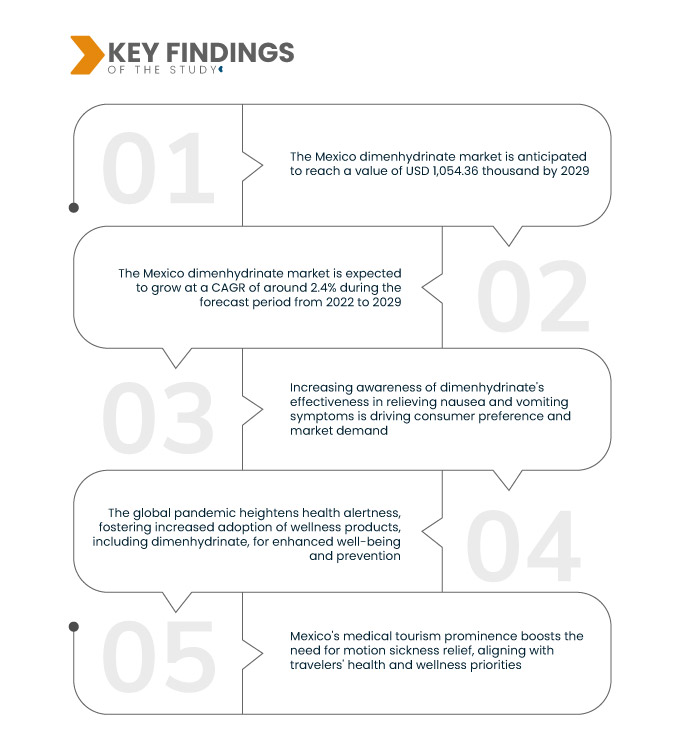
Data Bridge Market Research analyses that the Mexico Dimenhydrinate Market, which was USD 872.14 thousand in 2021, is expected to reach USD 1,054.36 thousand by 2029 and is expected to undergo a CAGR of 2.4% during the forecast period from 2022 to 2029. The expanding travel industry in Mexico propels a heightened need for motion sickness relief. As travel activities increase, there is a growing demand for products such as dimenhydrinate, known for its effectiveness in alleviating travel-induced nausea and vomiting symptoms.
Key Findings of the Study
Over-the-counter availability is expected to drive the market's growth rate
The over-the-counter availability of dimenhydrinate in Mexico significantly propels its market growth by enhancing consumer convenience. With easy accessibility, individuals can purchase the product without a prescription, facilitating a swift response to motion sickness or related symptoms. This accessibility caters to immediate consumer needs and contributes to increased sales and widespread adoption, as consumers can readily obtain dimenhydrinate for relief, thereby driving the market's expansion.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
202o (Customizable to 2014-2019)
|
|
Quantitative Units
|
Revenue in USD Thousand, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Prescription Type (Over-the-Counter (OTC) and Prescription Drugs), Application (Nausea, Vomiting, Dizziness, Insomnia, Allergies, and Others), Route of Administration (Oral and Parenteral), Dosage Form (Tablets, Chewables, Syrup, Injections, Others), Product Type (Branded, Generic), Age (Adult, Geriatric, Child), Distribution Channel (Store Based Retail and Online Based Retail)
|
|
Market Players Covered
|
Johnson & Johnson SA de CV (Mexico), Prestige Consumer Healthcare Inc. (U.S.), CINFA LABORATORIES (a subsidiary of Mundipharma) (U.K.)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Mexico dimenhydrinate market is categorized into seven segments based on prescription type, application, route of administration, dosage form, product type, age, and distribution channel.
- On the basis of prescription type, the Mexico dimenhydrinate market is segmented into over-the-counter (OTC) and prescription drugs
- On the basis of application, the Mexico dimenhydrinate market is segmented into nausea, vomiting, dizziness, insomnia, allergies, and others
- On the basis of route of administration, the Mexico dimenhydrinate market is segmented into oral and parenteral
- On the basis of dosage form, the Mexico dimenhydrinate market is segmented into tablets, chewables, syrup, injections, and others
- On the basis of product type, the Mexico dimenhydrinate market is segmented into branded and generic
- On the basis of age, the Mexico dimenhydrinate market is segmented into adult, geriatric, and child
- On the basis of distribution channel, the Mexico dimenhydrinate market is segmented into store based retail and online based retail
Major Players
Data Bridge Market Research recognizes the following companies as the major Mexico dimenhydrinate market players in Mexico dimenhydrinate market are Johnson & Johnson SA de CV (Mexico), Prestige Consumer Healthcare Inc. (U.S.), CINFA LABORATORIES (a subsidiary of Mundipharma) (U.K.)
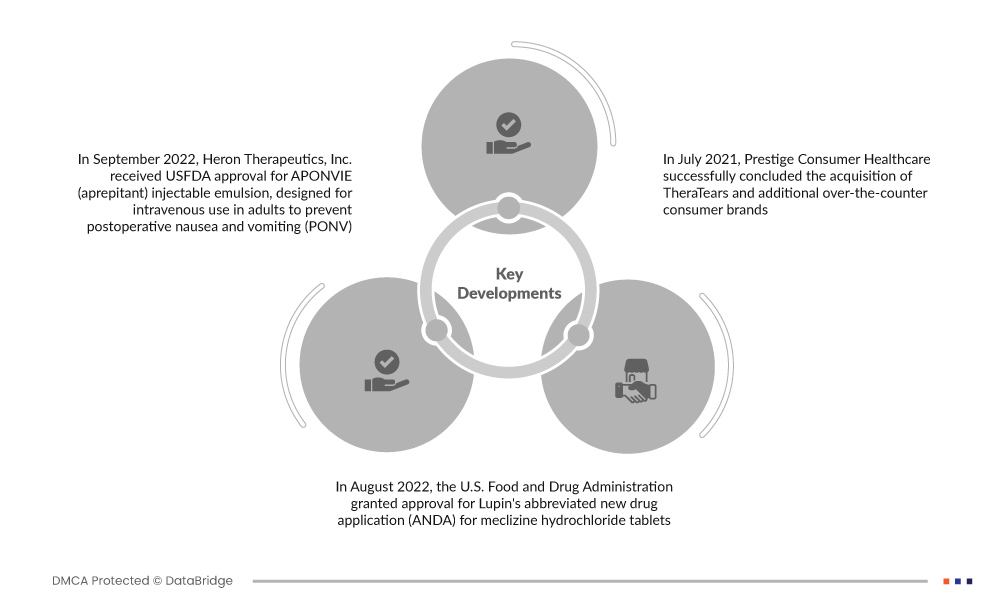
Market Developments
- In September 2022, Heron Therapeutics, Inc. received USFDA approval for APONVIE (aprepitant) injectable emulsion, designed for intravenous use in adults to prevent postoperative nausea and vomiting (PONV). This regulatory milestone signifies a significant advancement in addressing perioperative complications, offering a valuable solution to enhance the postoperative experience for patients
- In August 2022, the U.S. Food and Drug Administration approved Lupin's abbreviated new drug application (ANDA) for meclizine hydrochloride tablets (12.5 mg, 25 mg, and 50 mg). These tablets are designed to prevent and manage motion sickness-induced nausea, vomiting, and dizziness, along with addressing vertigo stemming from ear-related issues
- In July 2021, Prestige Consumer Healthcare successfully concluded the acquisition of TheraTears and additional over-the-counter consumer brands. This strategic move aims to boost the company's manufacturing capabilities within the U.S., fostering increased production efficiency. The acquisition is anticipated to contribute to revenue growth, aligning with Prestige Consumer Healthcare's commitment to expanding its market presence and product offerings
For more detailed information about the Mexico dimenhydrinate market report, click here – https://www.databridgemarketresearch.com/reports/mexico-dimenhydrinate-market