The anti-snoring devices and snoring surgery market showcases a spectrum of product types including oral appliances, nasal devices, position control devices, chin straps, EPAP therapy devices, and CPAP devices. Mandibular advancement devices (MAD), tongue stabilizing devices (TSD), and other innovative solutions constitute the diverse device types. This array underscores the market's commitment to addressing snoring through varied mechanisms, offering users a range of effective options tailored to individual needs, heralding a new era in sleep-related healthcare.
Access Full Report @ https://www.databridgemarketresearch.com/reports/north-america-anti-snoring-devices-and-snoring-surgery-market
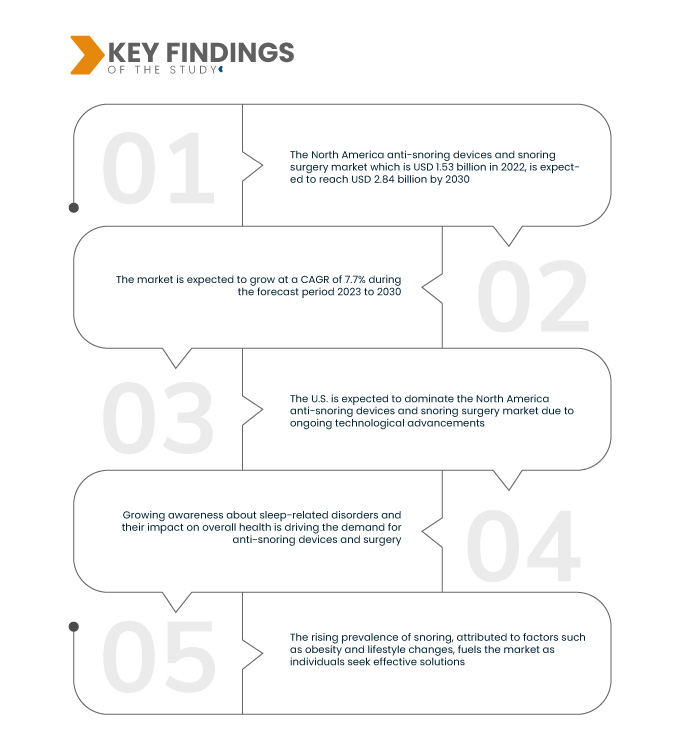
Data Bridge Market Research analyses that the North America Anti-Snoring Devices and Snoring Surgery Market which is USD 1.53 billion in 2022, is expected to reach USD 2.84 billion by 2030, at a CAGR of 7.7% during the forecast period 2023 to 2030. Elevated investments in research and development drive the anti-snoring devices and snoring surgery market by fostering the creation of advanced solutions. This commitment results in the continual introduction of innovative anti-snoring technologies, expanding the market, and addressing evolving consumer needs for effective and improved treatments.
Key Findings of the Study
Personalized healthcare is expected to drive the market's growth rate
The anti-snoring devices and snoring surgery market thrives on the trend toward personalized healthcare, emphasizing tailored solutions for individual needs. This shift recognizes the diverse nature of snoring causes and preferences among consumers. By offering customized anti-snoring devices and surgical approaches, the market enhances effectiveness and patient satisfaction. This driver underscores a commitment to addressing the unique requirements of individuals, contributing to the market's growth and evolution in meeting varied healthcare demands.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Product Type (Oral Appliances/Mouthpieces, Nasal Devices, Position Control Devices, Chin Straps, EPAP Therapy Devices and Continuous Positive Airway Pressure (CPAP) Devices), Device Type (Mandibular Advancement Devices (MAD), Tongue Stabilizing Device (TSD), Continuous Positive Airway Pressure (CPAP) Devices, Others), Surgery Type (Uvulopalatopharyngoplasty UPPP, Tonsillectomy and Adenoidectomy Surgery, Maxillo-mandibular and Genioglossus Advancement Surgeries, Laser-Assisted Uvulopalatoplasty LAUP, Radiofrequency Ablation, Pillar Procedure, Palatal Stiffening Procedures, Injection Snoreplasty and Others), Mode of Purchase (Over the Counter and Prescription Based), Gender (Male and Female), End User (Hospitals, Community Healthcare, Sleep Clinics, Home Healthcare and Others), Distribution Channel (Direct Tenders, Retail Sales and Online Sales)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America.
|
|
Market Players Covered
|
Apnea Sciences (U.S.), The Pure Sleep Company (U.S), KELSO & COMPANY (U.S.), ZQuiet (U.S.), GSK plc (U.K.), MPowrx (U.S.), ZYPPAH (U.S.), LivaNova PLC (U.K.), Koninklijke Philips N.V. (Netherlands), Fisher & Payker Healthcare Limited (New Zealand), Tomed GmbH (Germany), SomnoMed (Australia), Meditas Ltd. (U.K.), AirAvant Medical (U.S.), AccuMED Corp. (U.S.), General Electric Company (U.S.), Medtronic (Ireland), ResMed (U.S.), Tomed GmbH (Germany)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The North America anti-snoring devices and snoring surgery market is segmented on the basis of product type, surgery type, device type, mode of purchase, gender, end user and distribution channel.
- On the basis of product type, the North America anti-snoring devices and snoring surgery market is segmented into oral appliances/mouthpieces, nasal devices, position control devices, chin straps, EPAP therapy devices and continuous positive airway pressure (CPAP) devices
- On the basis of surgery type, the North America anti-snoring devices and snoring surgery market is segmented into uvulopalatopharyngoplasty UPPP, tonsillectomy and adenoidectomy surgery, maxillo-mandibular and genioglossus advancement surgeries, laser-assisted uvulopalatoplasty LAUP, radiofrequency ablation, pillar procedure, palatal stiffening procedures, injection snoreplasty and others
- On the basis of device type, the North America anti-snoring devices and snoring surgery market is segmented into mandibular advancement devices (MAD), TONGUE STABILIZING DEVICE (TSD), continuous positive airway pressure (CPAP) devices, and others
- On the basis of mode of purchase, the North America anti-snoring devices and snoring surgery market is segmented into over the counter and prescription based
- On the basis of gender, the North America anti-snoring devices and snoring surgery market is segmented into male and female
- On the basis of end user, the North America anti-snoring devices and snoring surgery market is segmented into hospitals, community healthcare, sleep clinics, home healthcare and others
- On the basis of distribution channel, the North America anti-snoring devices and snoring surgery market is segmented into direct tenders, retail sales and online sales
Major Players
Data Bridge Market Research recognizes the following companies as the North America anti-snoring devices and snoring surgery market players in North America anti-snoring devices and snoring surgery market are Apnea Sciences (U.S.), The Pure Sleep Company (U.S), KELSO & COMPANY (U.S.), ZQuiet (U.S.), GSK plc (U.K.), MPowrx (U.S.), ZYPPAH (U.S.), LivaNova PLC (U.K.).
Market Developments
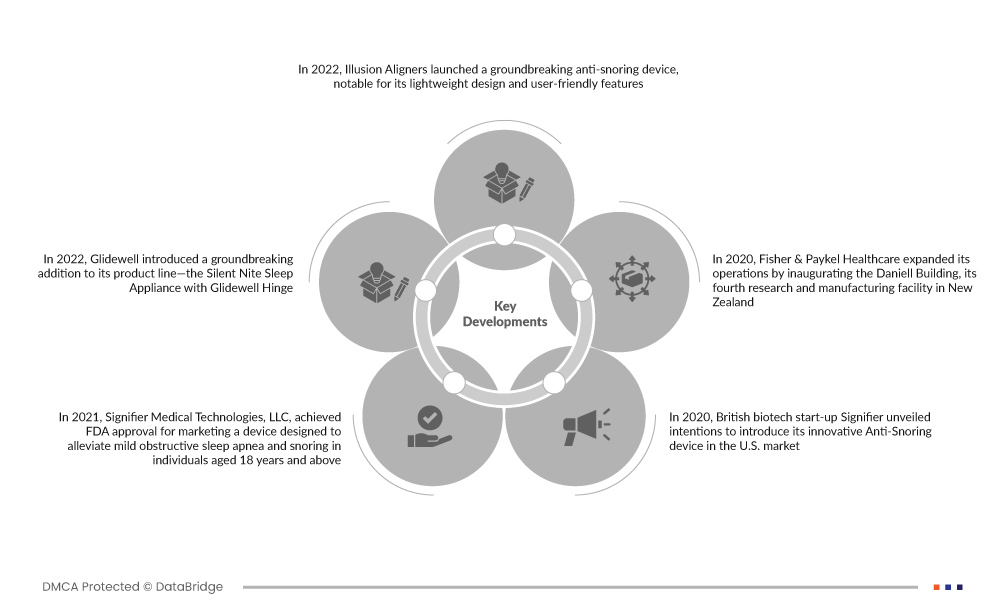
- In 2022, Illusion Aligners launched a groundbreaking anti-snoring device, notable for its lightweight design and user-friendly features. This oral device not only effectively addresses symptoms of obstructive sleep apnea (OSA) but also enhances overall sleep quality, reduces fatigue, and improves the user's quality of life. Illusion Aligners' innovation represents a significant step forward in providing a simple yet impactful solution for sleep-related issues
- In 2022, Glidewell introduced a groundbreaking addition to its product line—the Silent Nite Sleep Appliance with Glidewell Hinge. Specifically designed to address snoring and obstructive sleep apnea (OSA), this innovative device showcases advanced technology for mandibular advancement. With its unique Glidewell Hinge, the appliance offers an effective solution to enhance sleep quality and alleviate OSA symptoms, marking a significant advancement in sleep disorder treatment
- In 2021, Signifier Medical Technologies, LLC, achieved FDA approval for marketing a device designed to alleviate mild obstructive sleep apnea and snoring in individuals aged 18 years and above. This regulatory milestone signifies the device's safety and efficacy, providing patients with a non-invasive solution to address sleep-related issues and contributing to the advancement of accessible and effective sleep therapies.
- In 2020, British biotech start-up Signifier unveiled intentions to introduce its innovative Anti-Snoring device in the U.S. market. The company set an ambitious funding goal of $100 million for the project. This strategic move reflected Signifier's commitment to addressing the demand for advanced anti-snoring solutions, showcasing the company's confidence in the device's potential impact and its dedication to securing substantial support for market entry and growth
- In 2020, Fisher & Paykel Healthcare expanded its operations by inaugurating the Daniell Building, its fourth research and manufacturing facility in New Zealand. This strategic development positions the company to advance its efforts in researching and producing cutting-edge healthcare solutions, particularly in the field of sleep disorders. The new facility reflects Fisher & Paykel Healthcare's commitment to innovation and underscores its capacity to deliver leading products for improved sleep-related healthcare
Regional Analysis
Geographically, the countries covered in the North America anti-snoring devices and snoring surgery market report are U.S., Canada and Mexico in North America.
As per Data Bridge Market Research analysis:
U.S. is expected to dominate the North America anti-snoring devices and snoring surgery market in the forecast period 2023-2030
The U.S. is expected to dominate the North America anti-snoring devices and snoring surgery market due to ongoing technological advancements. The development of innovative, customizable devices designed for both men and women contribute to the country's dominance. This trend reflects a commitment to addressing diverse snoring concerns through cutting-edge solutions, positioning the U.S. as a key player in shaping the market landscape during the forecast period.
For more detailed information about the North America anti-snoring devices and snoring surgery market report, click here – https://www.databridgemarketresearch.com/reports/north-america-anti-snoring-devices-and-snoring-surgery-market