Alpha-1 antitrypsin deficiency (AATD) augmentation therapy is a medical intervention designed to address the genetic condition where the body produces insufficient alpha-1 antitrypsin (AAT) protein. AAT protects the lungs from damage caused by enzymes. Augmentation therapy involves infusing purified AAT into the bloodstream to restore adequate levels. Through enhancing AAT levels, this therapy aims to slow down the progression of lung disease in individuals with AATD, ultimately improving respiratory function and overall quality of life.
Access Full Report @ https://www.databridgemarketresearch.com/reports/us-alpha-1-antitrypsin-deficiency-aatd-augmentation-therapy-market
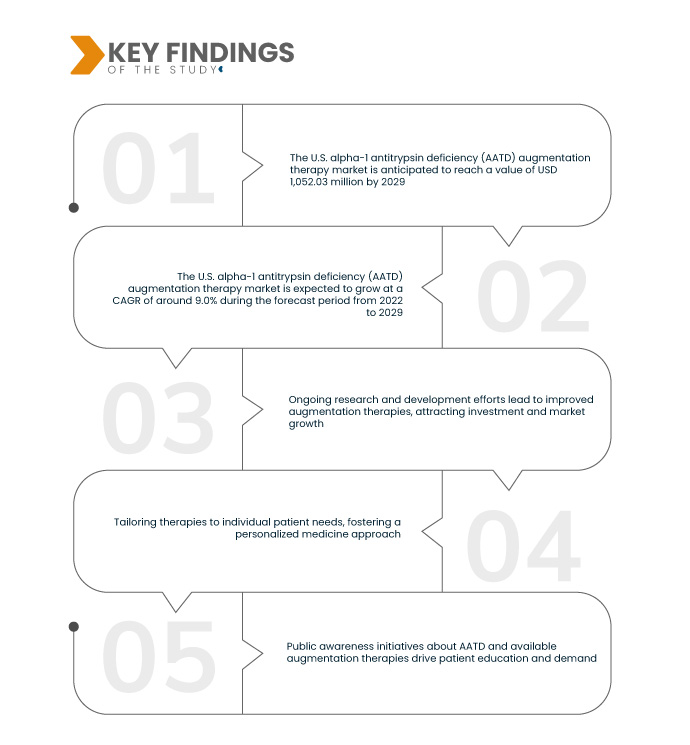
Data Bridge Market Research analyses that the U.S. Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market which was USD 563.24 million in 2021, is expected to USD 1,052.03 million by 2029, and is expected to undergo a CAGR of 9.0% during the forecast period from 2022-2029. Government initiatives, subsidies, and support for rare disease treatments, such as AATD augmentation therapy, positively influence market growth, fostering accessibility and affordability for patients in need.
Key Findings of the Study
Escalating respiratory diseases is expected to drive the market's growth rate
The escalating global prevalence of respiratory diseases, particularly those linked to alpha-1 antitrypsin deficiency (AATD), such as chronic obstructive pulmonary disease (COPD), intensifies the demand for efficacious treatments. Augmentation therapy becomes crucial in addressing the rising incidence of respiratory conditions, underlining the imperative for advanced therapeutic interventions to alleviate the impact of AATD on lung health. This trend propels the market for AATD augmentation therapy as a pivotal component in managing respiratory disorders worldwide.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Drugs (Prolastin, Aralast NP, Zemaira/Respreeza, Glassia And Others), Gene Type (Type Pimz, Type Pims, Type Pizz And Others), Application (Lung Disease And Liver Disease), Population Type (Adults And Pediatric), End User (Hospitals, Specialty Clinics, Home Healthcare, And Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy And Others)
|
|
Market Players Covered
|
Grifols, S.A. (Spain), CSL (Australia), Kamada Pharmaceuticals (Israel), Takeda Pharmaceutical Company Limited: (Japan), LFB BIOMEDICAMENTS (France), Arrowhead Pharmaceuticals, Inc. (U.S.), Mereo BioPharma Group plc (U.K), Inhibrx, Inc. (U.S.), Centessa Pharmaceuticals (Z Factor) (U.S.), Intellia Therapeutics, Inc. (U.S.), Apic Bio (U.S.), Krystal Biotech (U.S.), Beam Therapeutics (U.S.), LOGICBIO THERAPEUTICS, INC. (U.S.) among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into drugs, gene type, application, population type, end user, and distribution channel.
- On the basis of drugs, the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into prolastin, aralast NP, zemaira/respreeza, glassia, and others
- On the basis of gene type, the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into type PiMZ, type PiMS, type PiZZ and others
- On the basis of application, the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into lung disease and liver disease
- On the basis of population type, the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into adults and pediatric
- On the basis of end user, the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into hospitals, specialty clinics, home healthcare, and others
- On the basis of distribution channel, the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others
Major Players
Data Bridge Market Research recognizes the following companies as the major U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market players in U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market are Grifols, S.A. (Spain), CSL (Australia), Kamada Pharmaceuticals (Israel), Takeda Pharmaceutical Company Limited: (Japan), LFB BIOMEDICAMENTS (France), Arrowhead Pharmaceuticals, Inc. (U.S.)
Market Developments
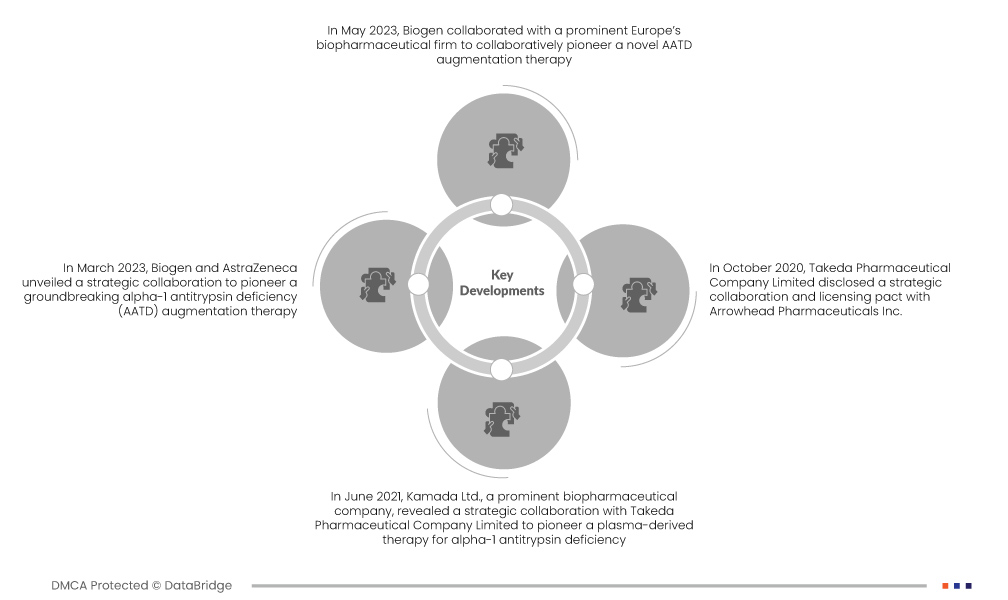
- In May 2023, Biogen collaborated with a prominent Europe’s biopharmaceutical firm to collaboratively pioneer a novel AATD augmentation therapy. This strategic alliance leverages their combined biotechnological prowess to advance treatment solutions for alpha-1 antitrypsin deficiency, aiming to meet unmet medical needs. The collaboration underscores a shared commitment to innovation, potentially broadening the market impact of their collaborative therapeutic efforts
- In March 2023, Biogen and AstraZeneca unveiled a strategic collaboration to pioneer a groundbreaking alpha-1 antitrypsin deficiency (AATD) augmentation therapy. This collaboration synergizes their collective expertise and resources to propel advancements in AATD treatment, heralding a new era in addressing the needs of affected individuals. The collaboration signifies a concerted effort to innovate and improve therapeutic options for AATD patients
- In June 2021, Kamada Ltd., a prominent biopharmaceutical company, revealed a strategic collaboration with Takeda Pharmaceutical Company Limited to pioneer a plasma-derived therapy for alpha-1 antitrypsin deficiency. Merging Takeda's expertise with Kamada's advanced technology, the collaboration aims to revolutionize AATD treatment, elevating patient outcomes. This collaboration underscores a joint commitment to advancing therapeutic solutions and fostering market expansion
- In October 2020, Takeda Pharmaceutical Company Limited disclosed a strategic collaboration and licensing pact with Arrowhead Pharmaceuticals Inc. The collaboration focuses on advancing ARO-AAT, an investigational RNA interference (RNAi) therapy in Phase 2 development for alpha-1 antitrypsin-associated liver disease (AATLD). This collaboration enhances Takeda's global footprint, showcasing a concerted effort to address AATLD through innovative therapeutic solutions
For more detailed information about the U.S. alpha-1 antitrypsin deficiency (AATD) augmentation therapy market report, click here – https://www.databridgemarketresearch.com/reports/us-alpha-1-antitrypsin-deficiency-aatd-augmentation-therapy-market